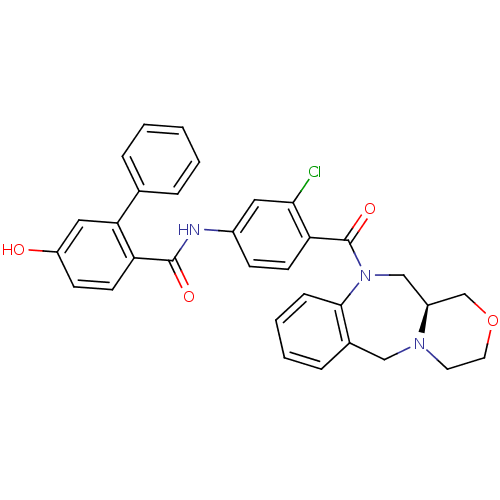
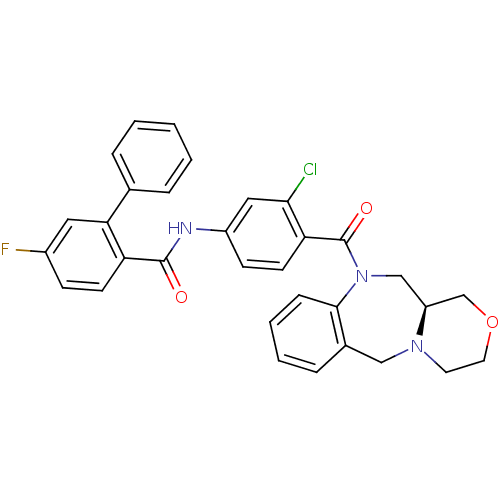
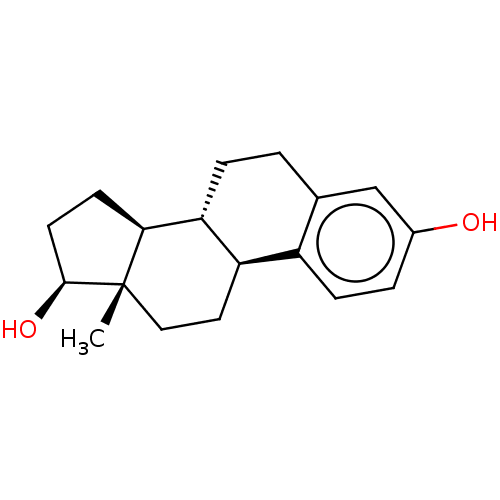
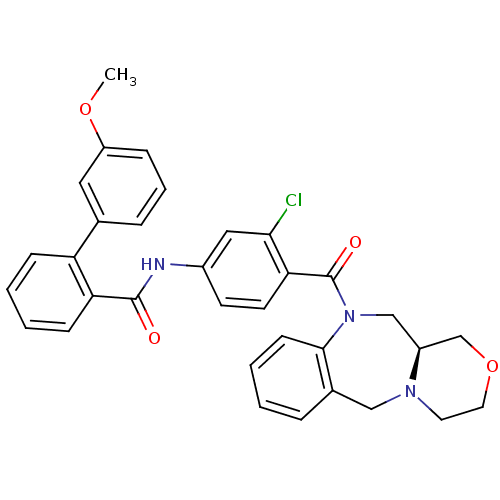
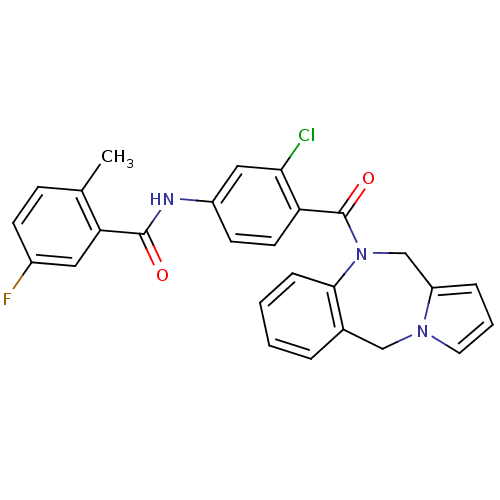
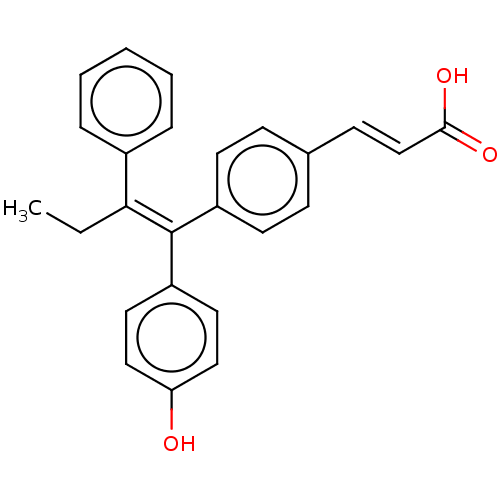
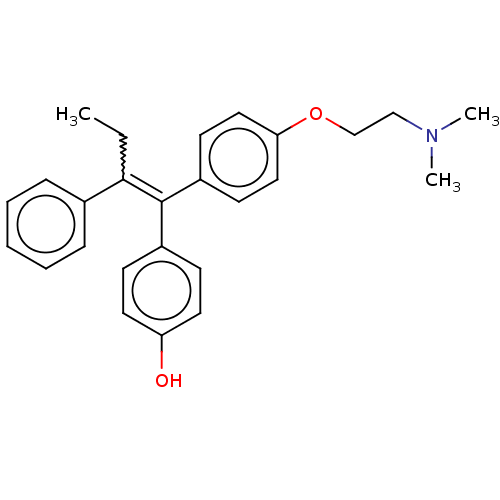
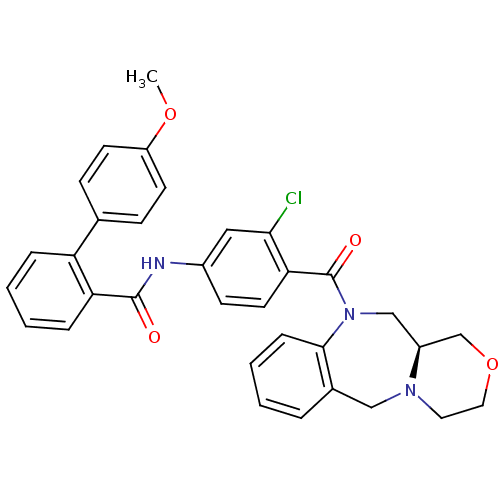
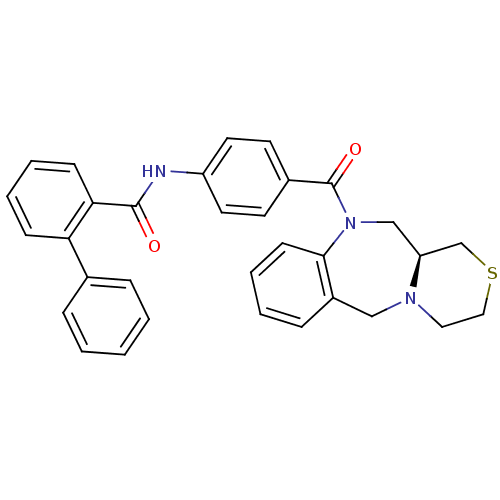
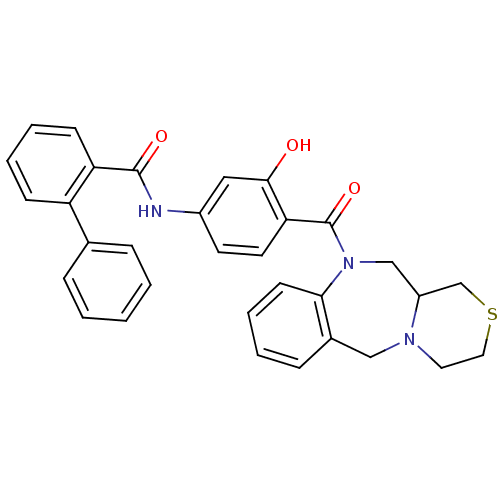
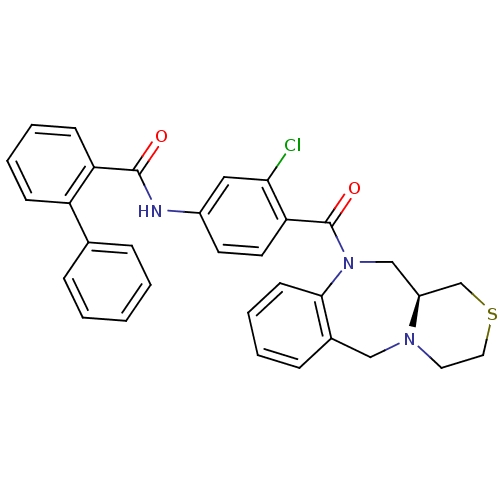
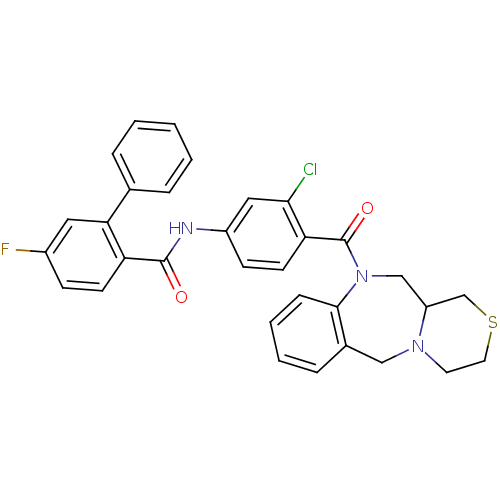
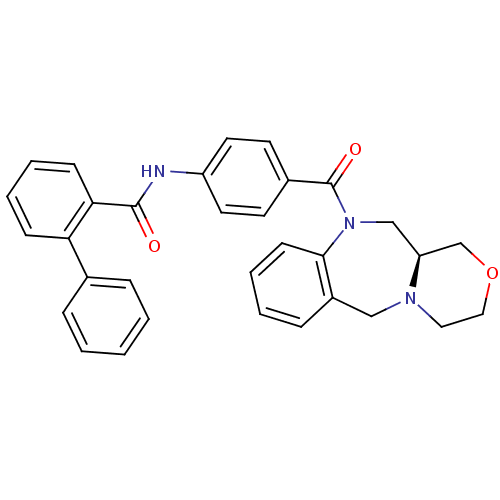
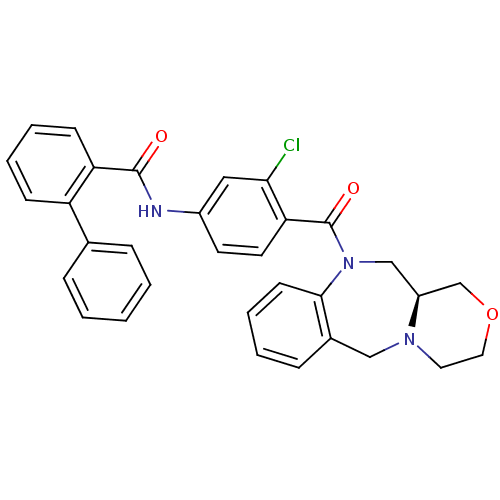
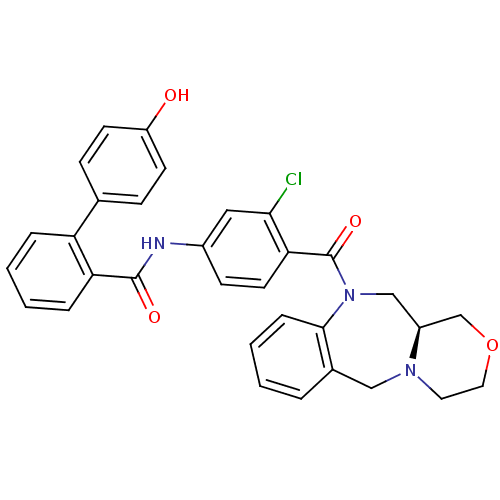
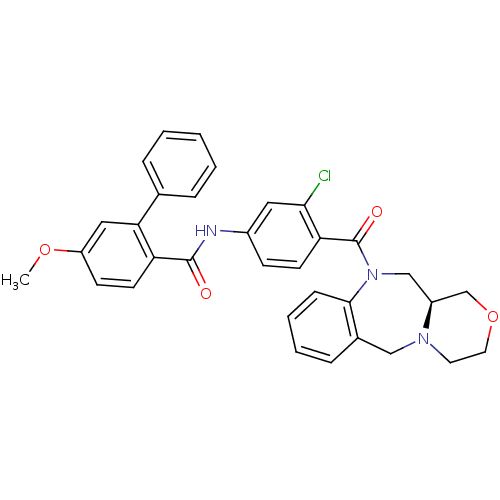
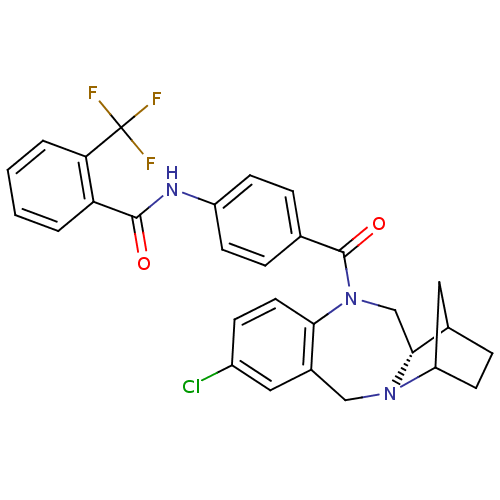
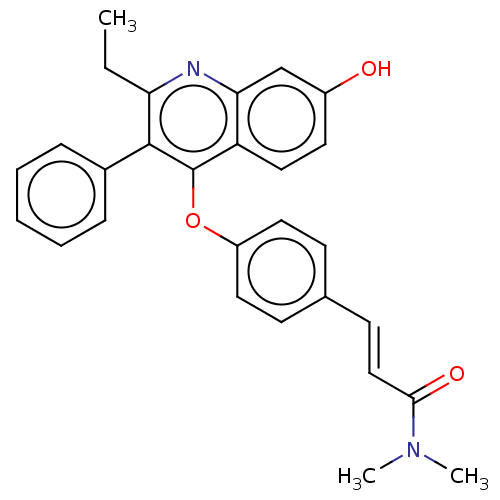
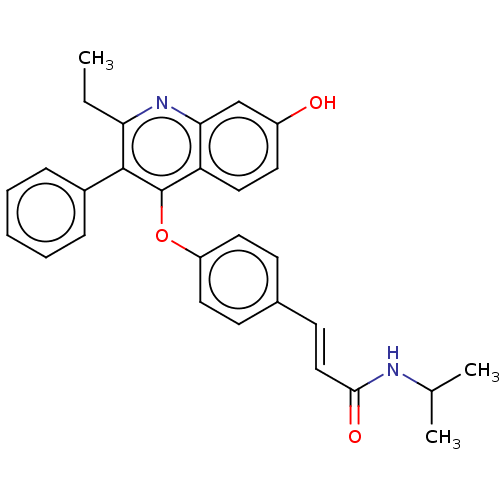
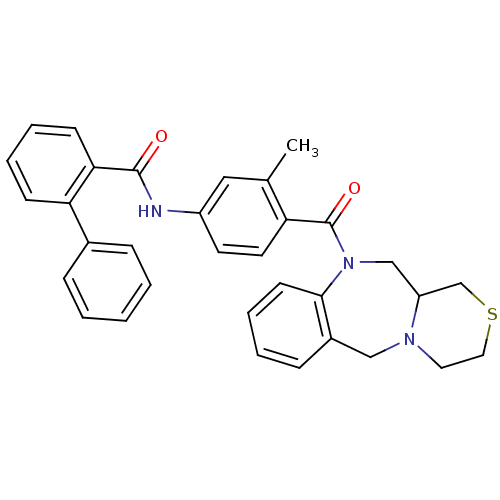
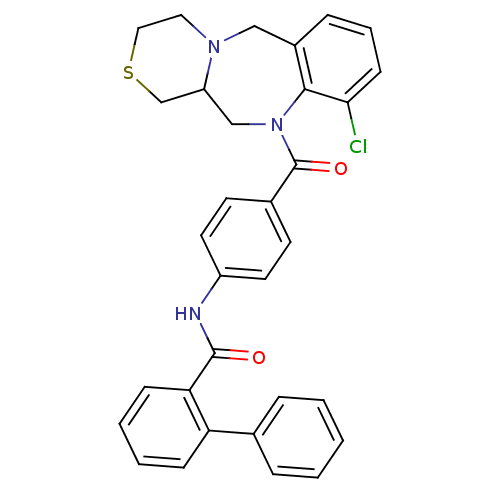
Affinity DataKi: 0.251nMAssay Description:Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-BMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
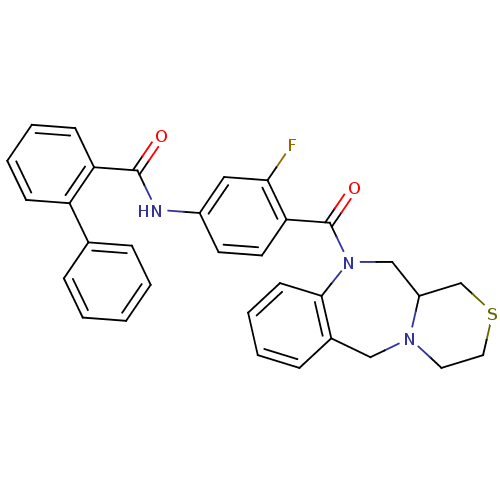
Affinity DataKi: 1.40nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
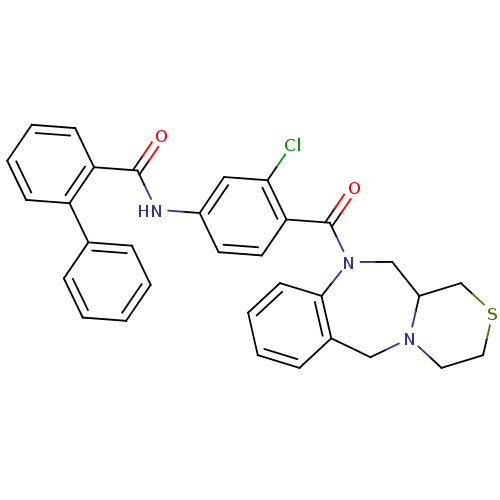
Affinity DataKi: 1.90nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
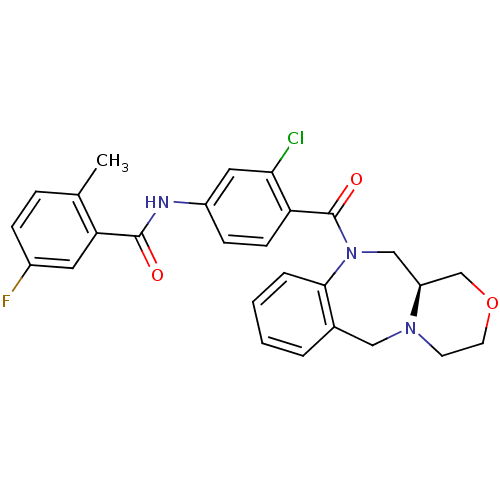
Affinity DataKi: 2nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V1a receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 7.90nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptorMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Glaxosmithkline Research & Development
Curated by ChEMBL
Glaxosmithkline Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of fluorescent ligand from binding domain of progesterone receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V1a receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V1a receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cellsMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Inhibition of 1 nM AVP-induced calcium mobilisation in cells expressing human vasopressin V1a receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)