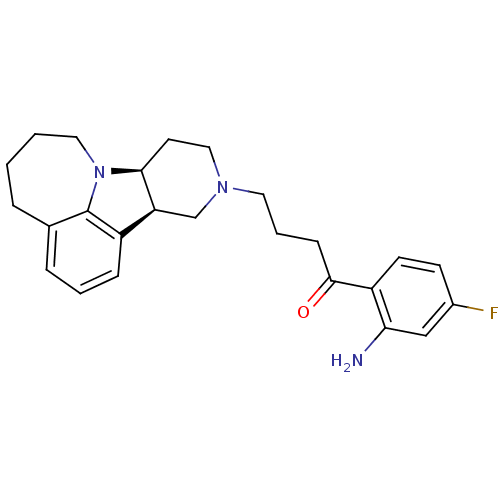
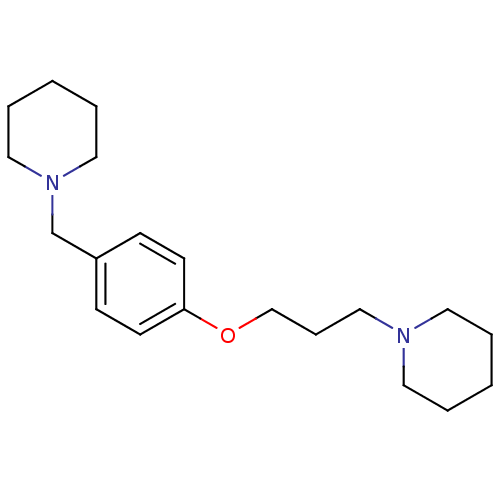
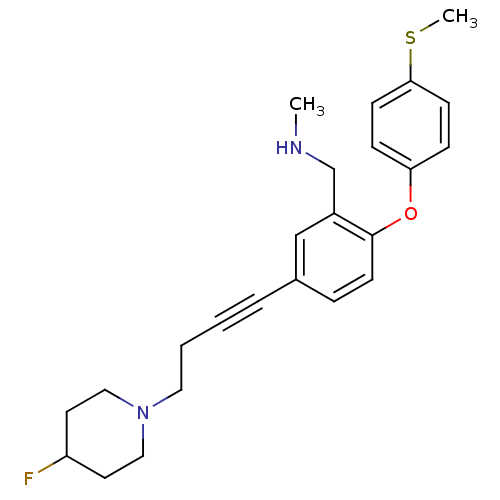
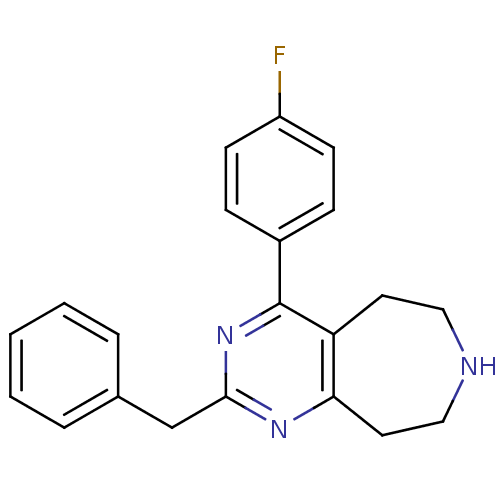
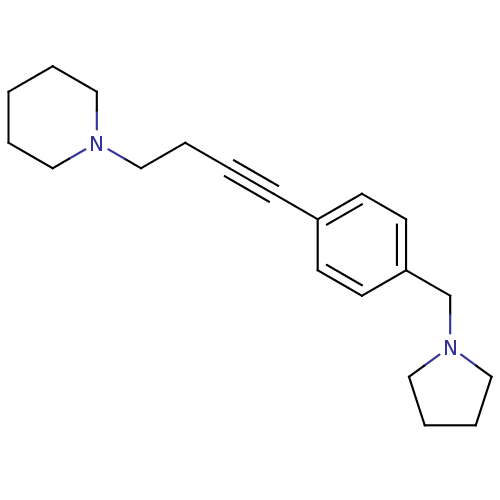
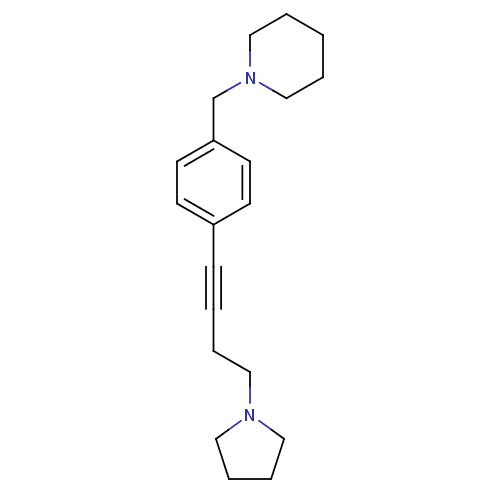
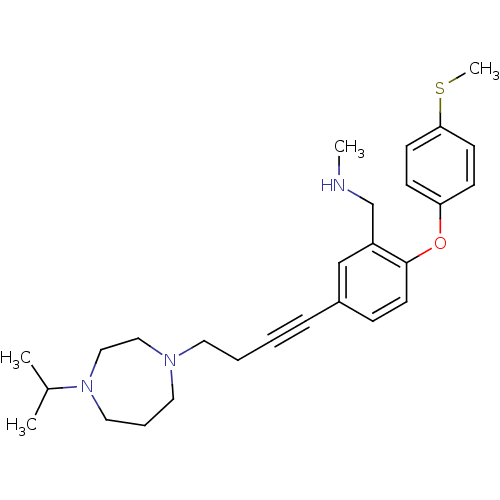
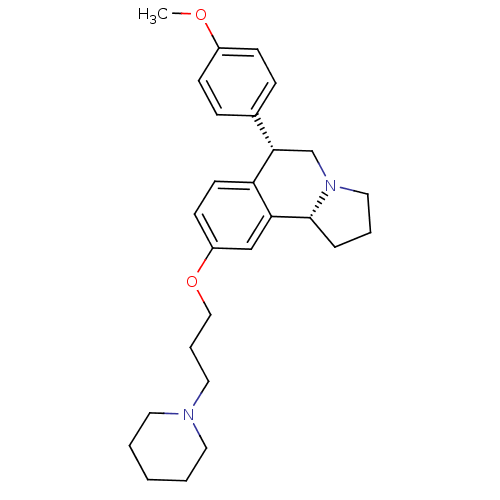
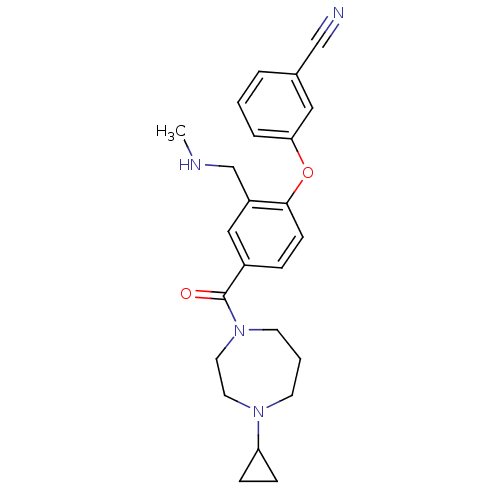
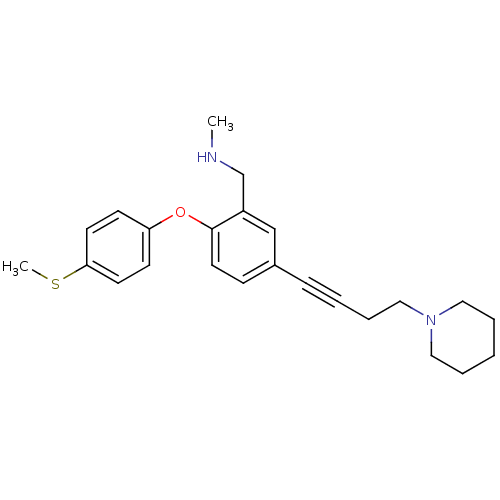
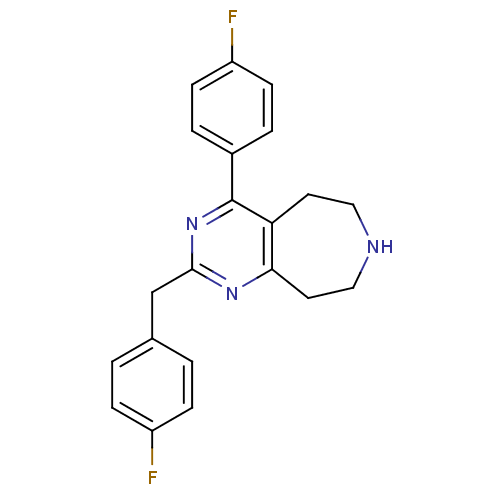
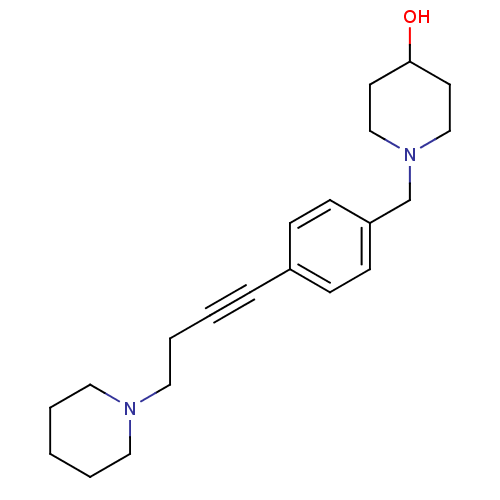
Affinity DataKi: 0.200nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
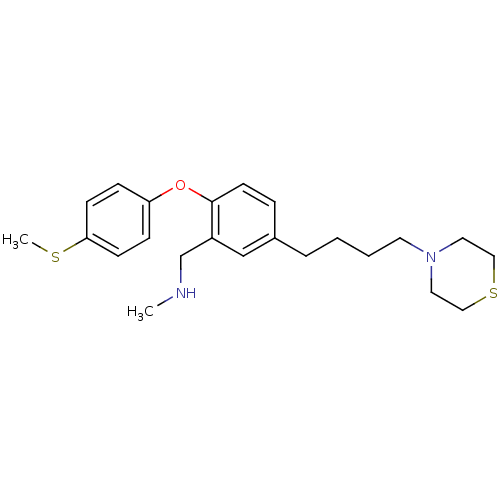
Affinity DataKi: 0.300nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.513nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.550nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -52.1kJ/molepH: 7.5 T: 2°CAssay Description:The kinase activity was determined by incubation of enzyme and its substrate, and test compound, in the presence ATP/[gamma-32P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.617nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.676nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Binding affinity towards DA D2 receptor using [3H]-N-methyl-spiperone as radioligand.More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to human SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.720nMAssay Description:Binding affinity at human histamine H3More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.724nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.730nMAssay Description:Displacement of [3H]BMS-725519 from human CB1 receptor expressed in CHO cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.759nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.776nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Binding affinity to human SERTMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand.More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human SERTMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.871nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.891nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Binding affinity to human SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair

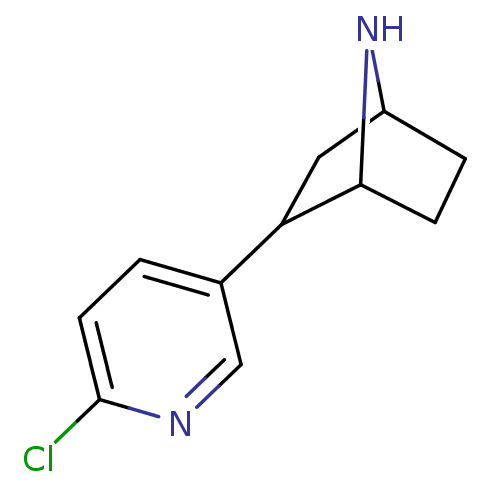
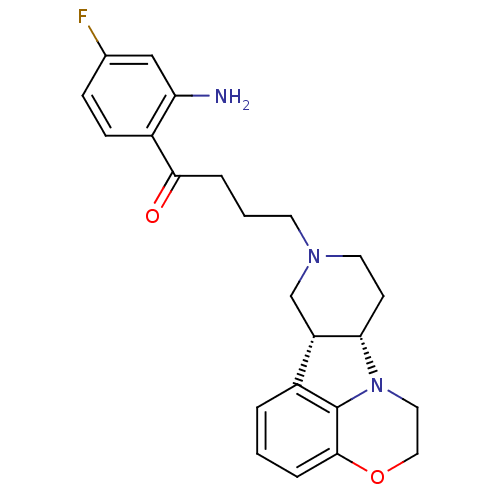
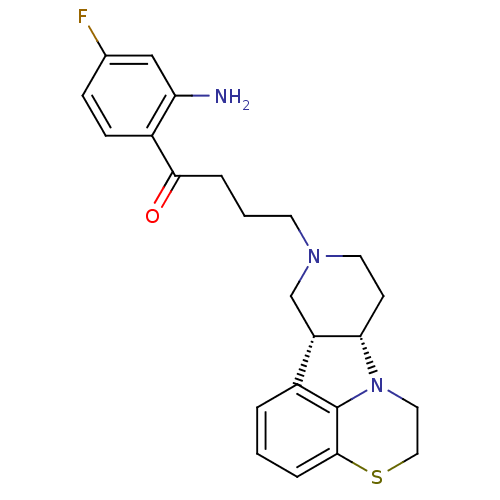
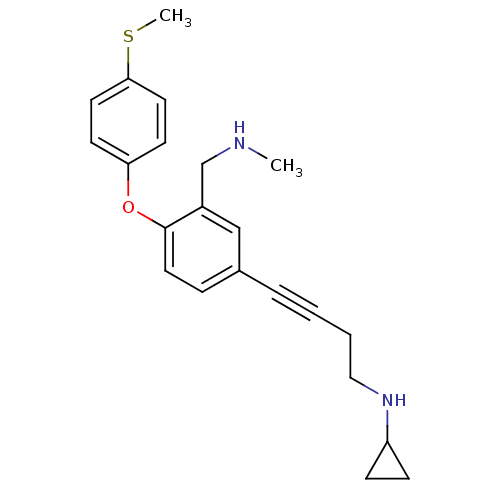
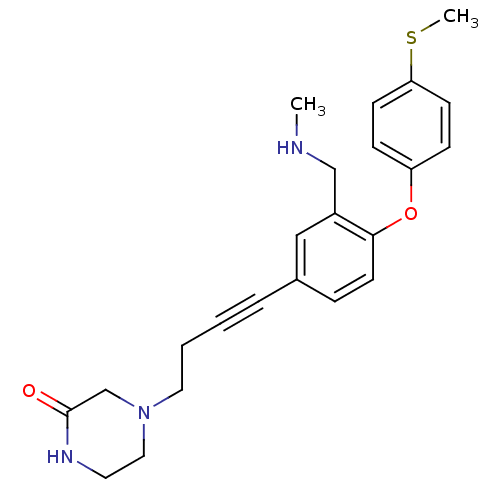
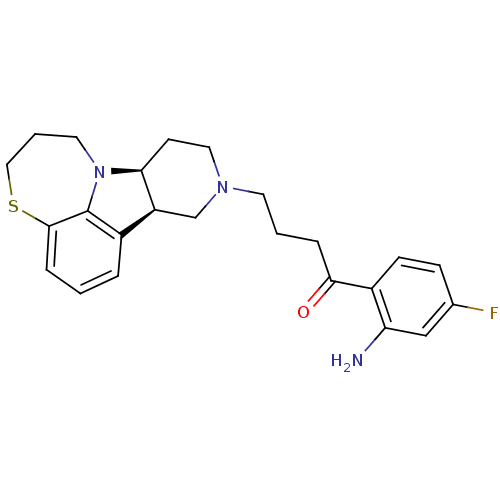

 3D Structure (crystal)
3D Structure (crystal)