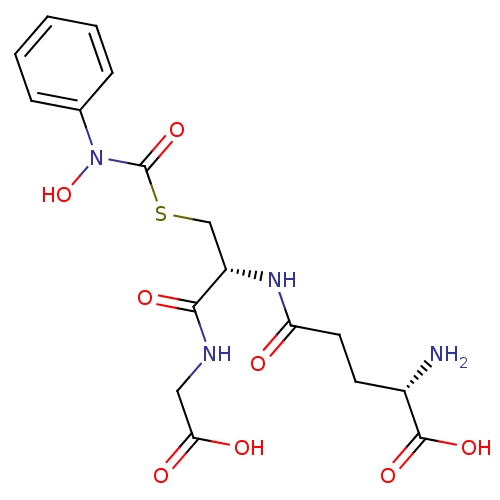
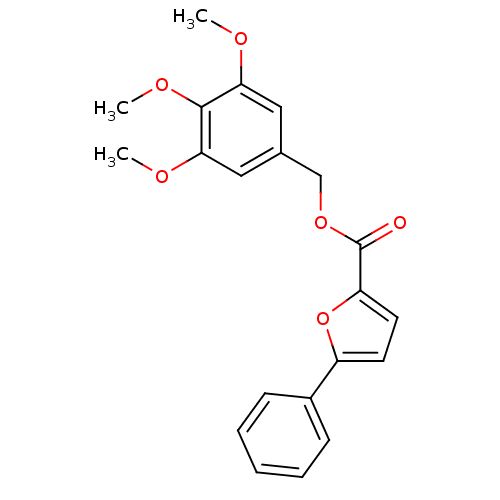
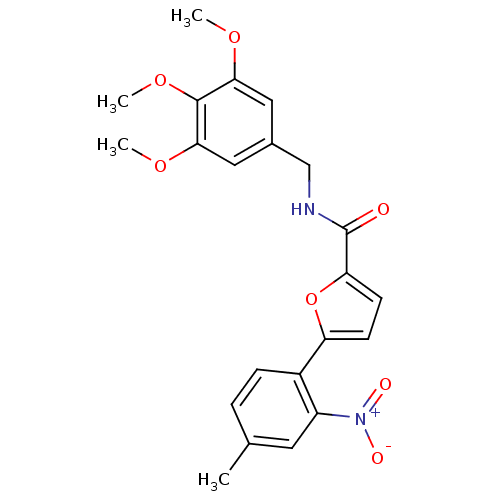
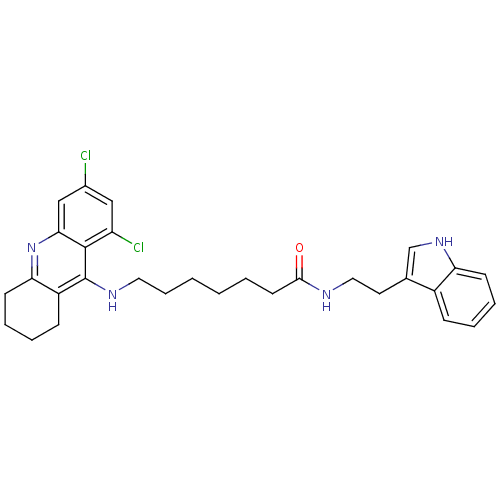
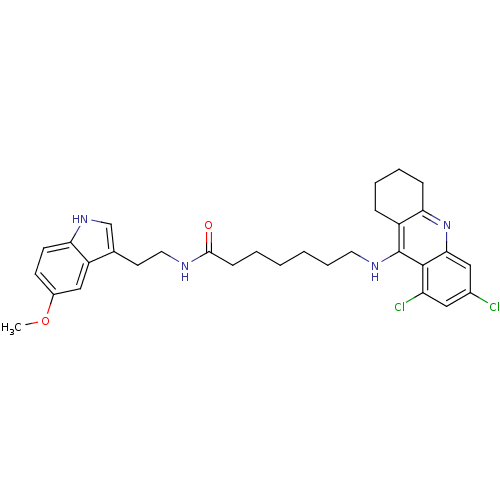
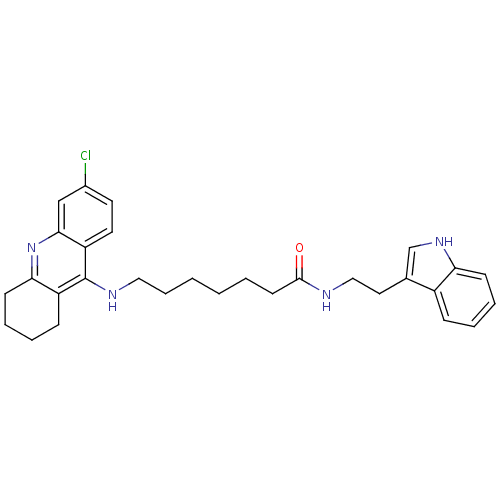
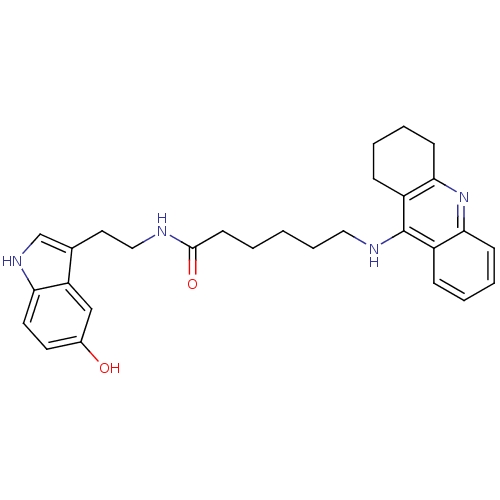
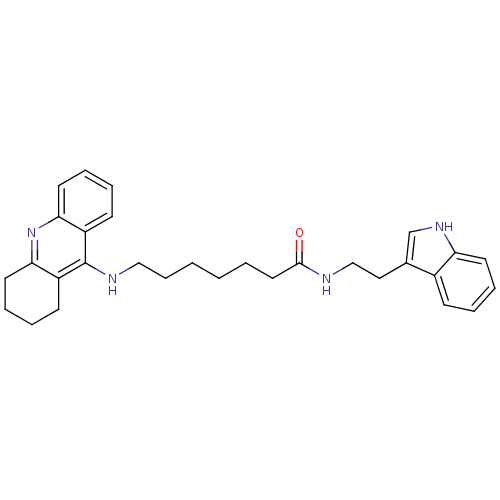
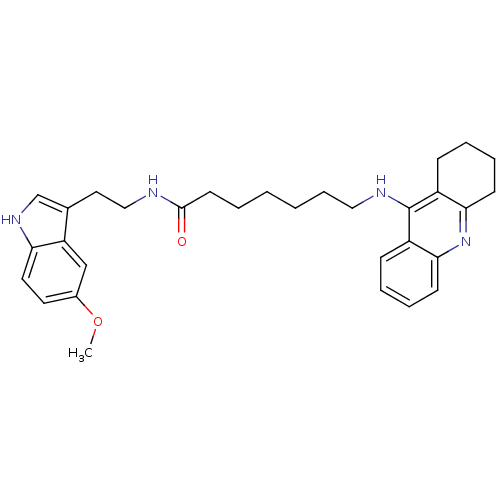
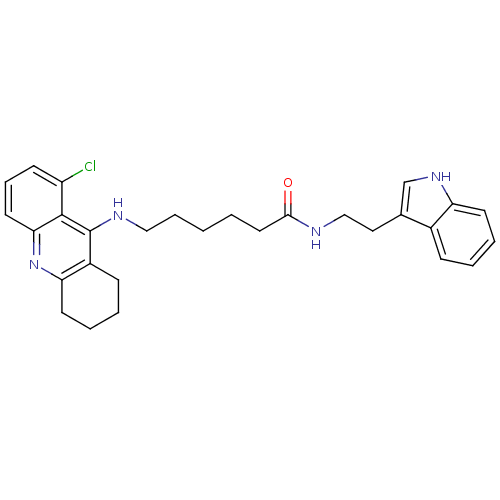
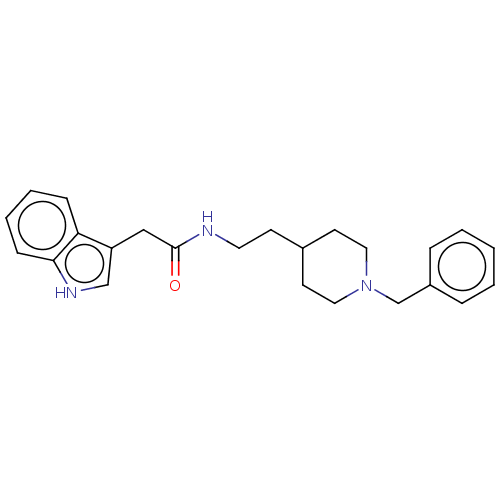
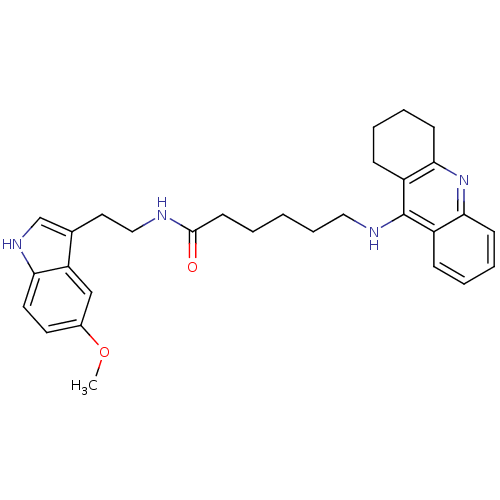
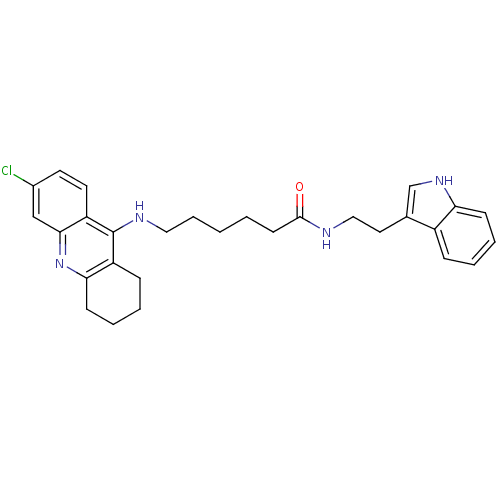
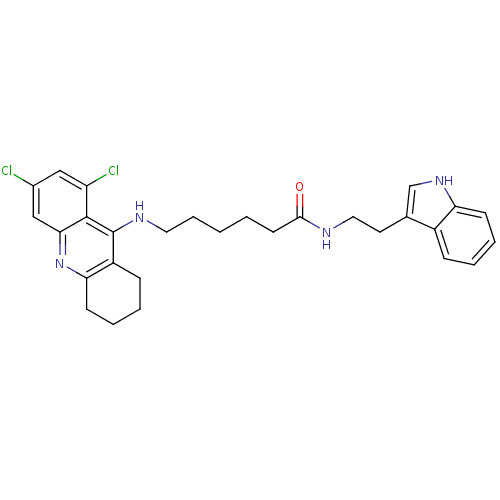
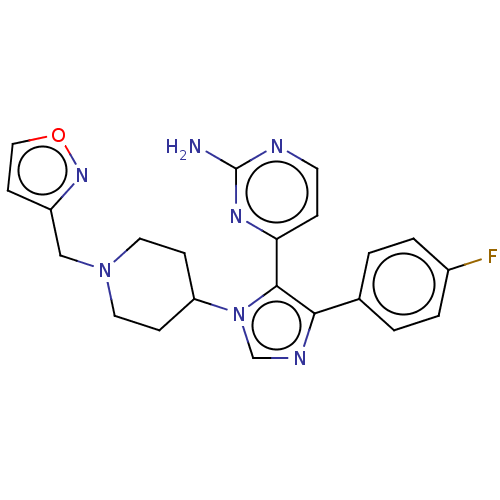
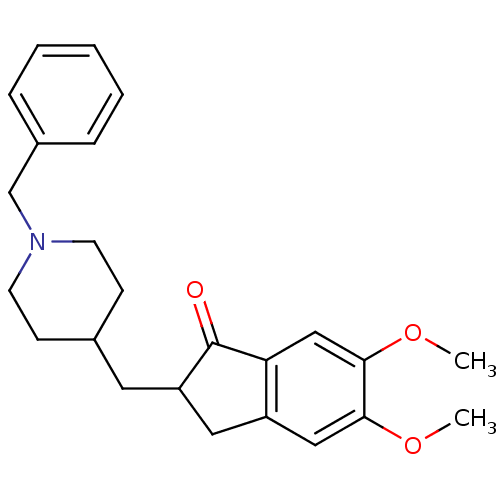
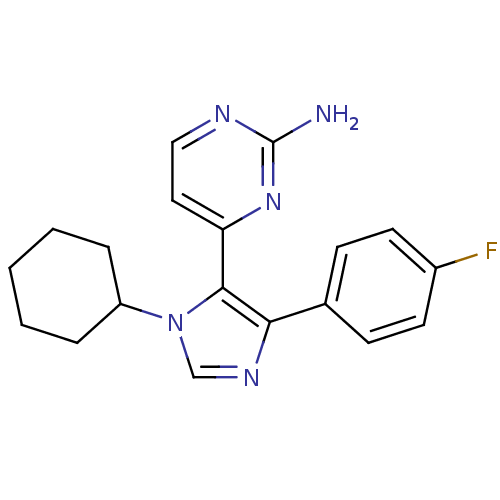
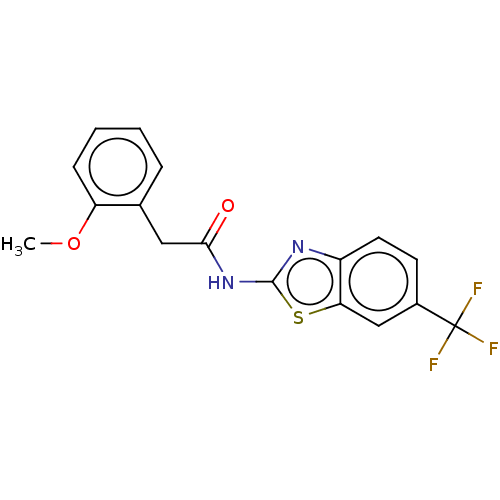
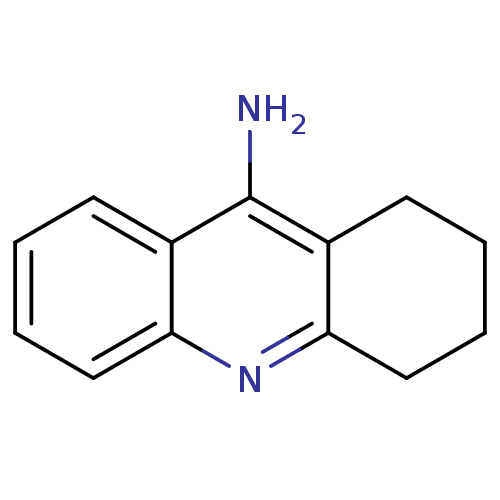
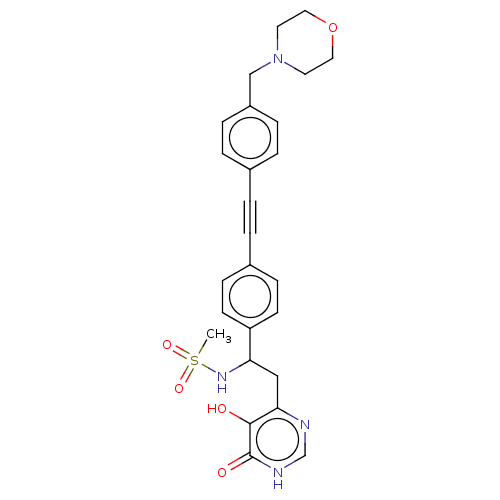
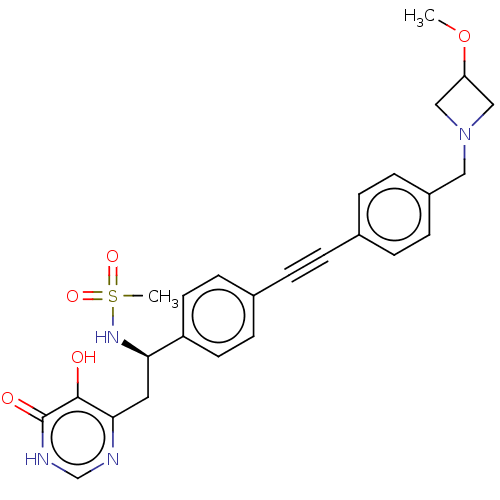
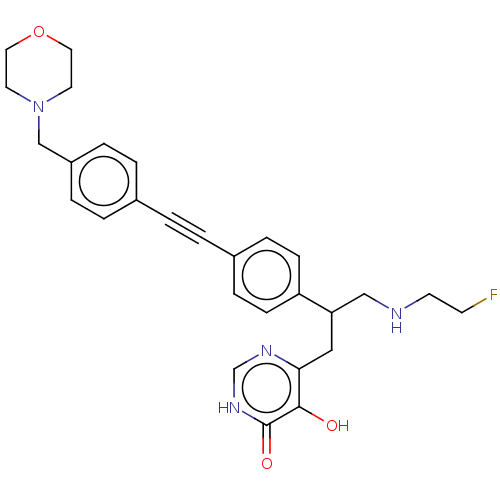
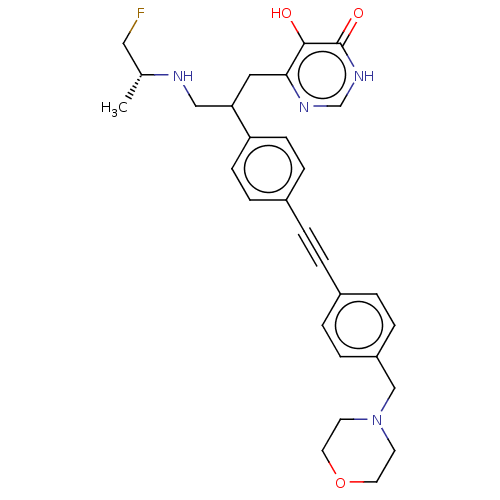
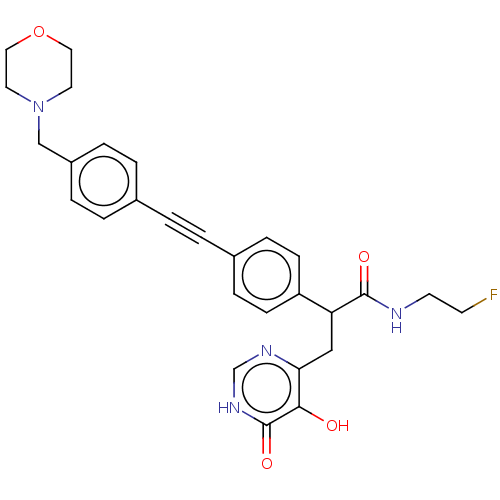
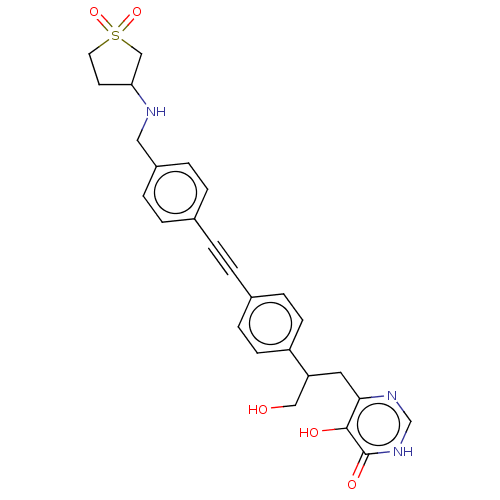
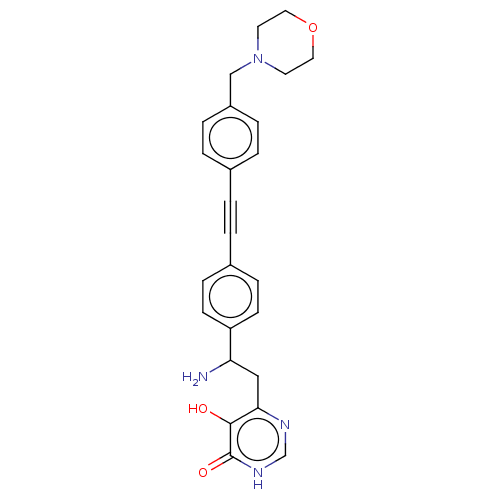
Affinity DataKi: 11nMAssay Description:Inhibition of human GLO1More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of human GLO1More data for this Ligand-Target Pair
Affinity DataKi: 230nMAssay Description:Inhibition of human GLO1More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A(Homo sapiens (Human))
Instituto De Qu£Mica M£Dica (Csic)
Curated by ChEMBL
Instituto De Qu£Mica M£Dica (Csic)
Curated by ChEMBL
Affinity DataKi: 5.91E+3nMAssay Description:Competitive inhibition of human recombinant PDE7A1 using 5 nM to 2 uM cAMP as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A(Homo sapiens (Human))
Instituto De Qu£Mica M£Dica (Csic)
Curated by ChEMBL
Instituto De Qu£Mica M£Dica (Csic)
Curated by ChEMBL
Affinity DataKi: 7.22E+3nMAssay Description:Competitive inhibition of human recombinant PDE7A1 using 5 nM to 2 uM cAMP as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.00800nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: 0.450nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMAssay Description:Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMAssay Description:Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMAssay Description:Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: 0.950nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibition of wild-type human CK1delta using PLSRTLpSVASLPGL as substrate incubated for 40 mins in presence of ATP by Kinase-Glo luminescence assayMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Instituto De Quimica Medica-Csic
Curated by ChEMBL
Instituto De Quimica Medica-Csic
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant GSK3betaMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.80nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of full-length human CK1epsilon expressed in Escherichia coli BL21-CodonPlus(DE3)-RIL competent cells using PLSRTLpSVASLPGL as substrate i...More data for this Ligand-Target Pair
Affinity DataIC50: 7.80nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.10nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant CK-1delta using casein as substrate after 60 mins by Kinase-Glo assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:IC50 values against E. coli LpxC were determined using a Rapid Fire MS assay as previously described J. Med. Chem. 2012, 55, 1662-1670.More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:IC50 values against E. coli LpxC were determined using a Rapid Fire MS assay as previously described J. Med. Chem. 2012, 55, 1662-1670.More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:IC50 values against E. coli LpxC were determined using a Rapid Fire MS assay as previously described J. Med. Chem. 2012, 55, 1662-1670.More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:IC50 values against E. coli LpxC were determined using a Rapid Fire MS assay as previously described J. Med. Chem. 2012, 55, 1662-1670.More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:IC50 values against E. coli LpxC were determined using a Rapid Fire MS assay as previously described J. Med. Chem. 2012, 55, 1662-1670.More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:IC50 values against E. coli LpxC were determined using a Rapid Fire MS assay as previously described J. Med. Chem. 2012, 55, 1662-1670.More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:IC50 values against E. coli LpxC were determined using a Rapid Fire MS assay as previously described J. Med. Chem. 2012, 55, 1662-1670.More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)