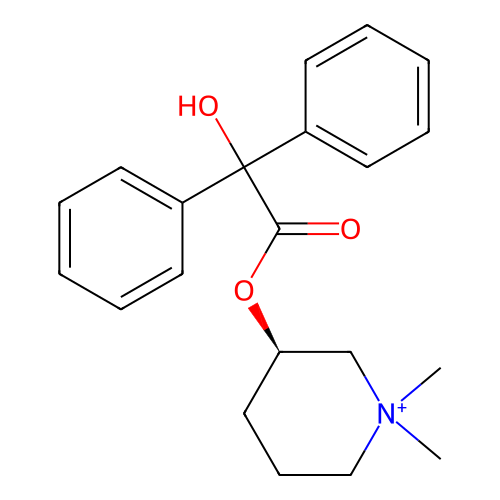
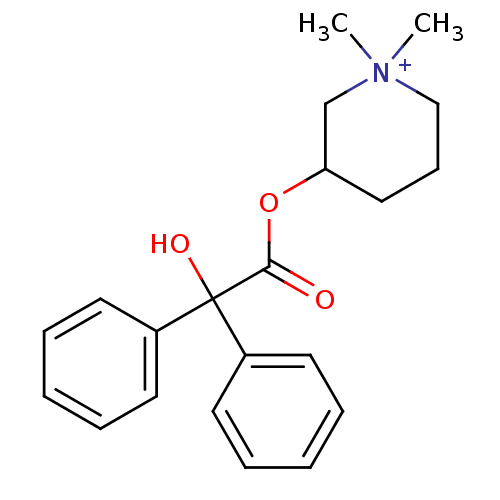
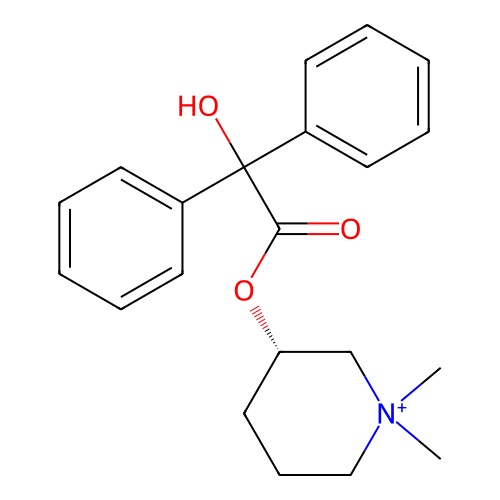
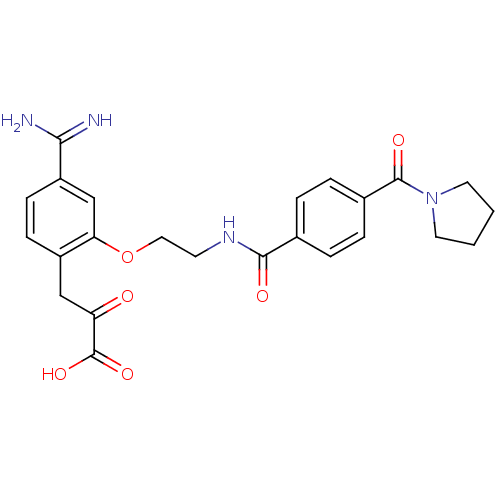
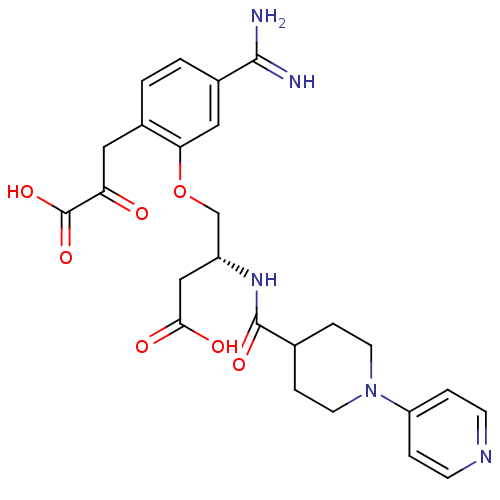
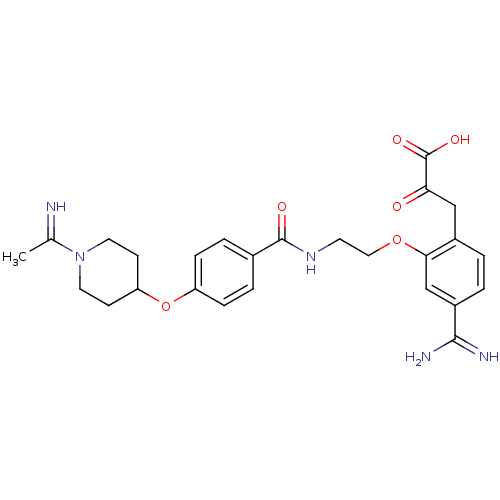
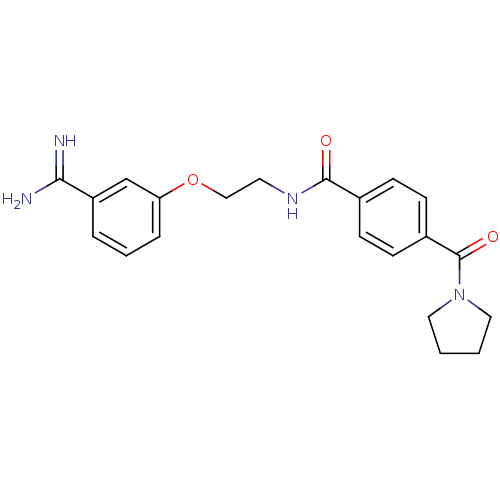
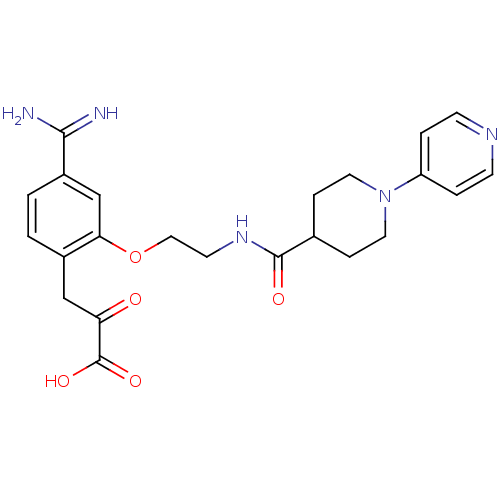
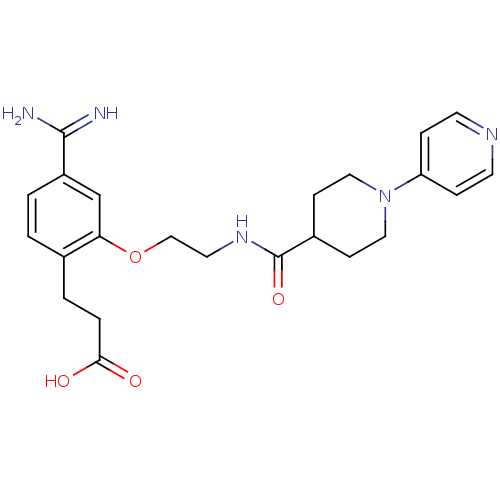
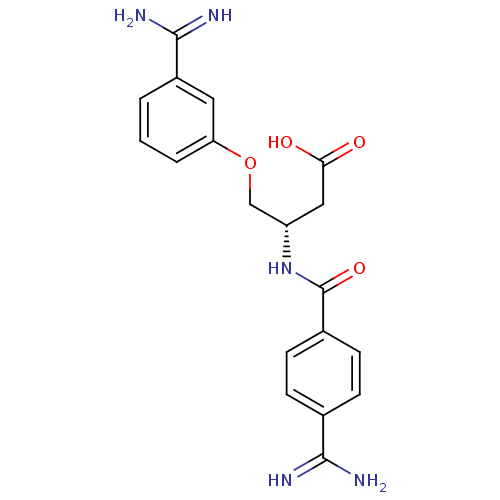
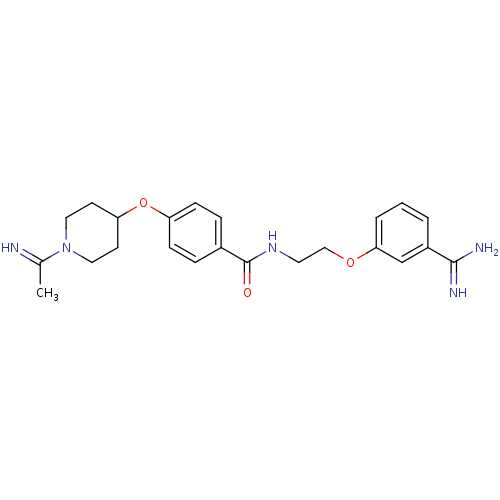
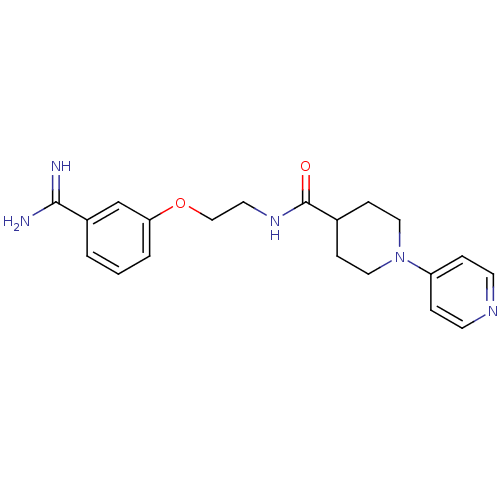
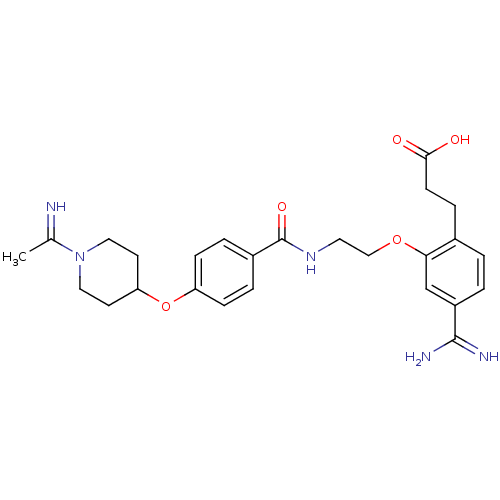
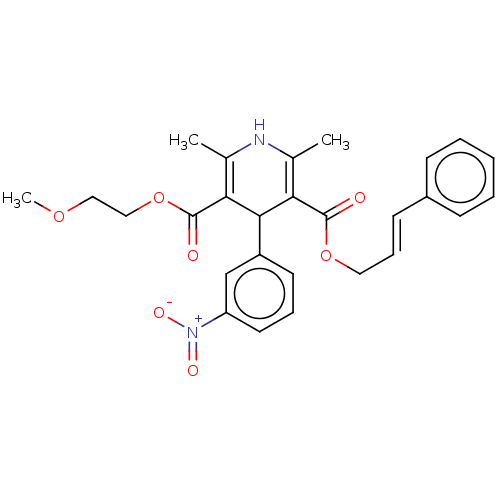
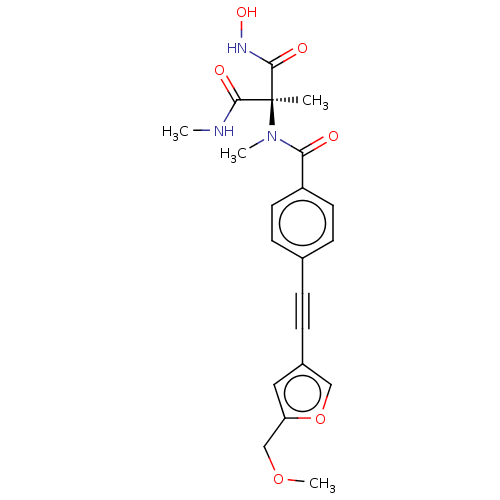
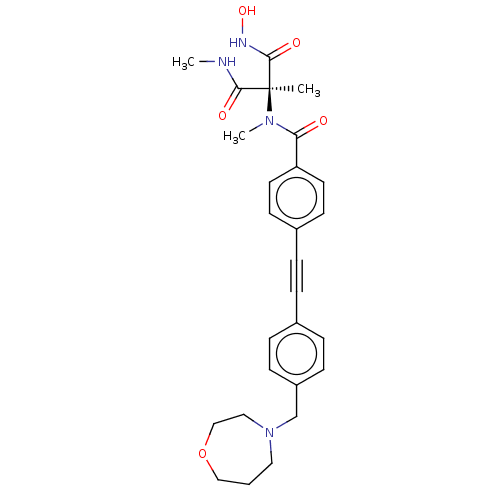
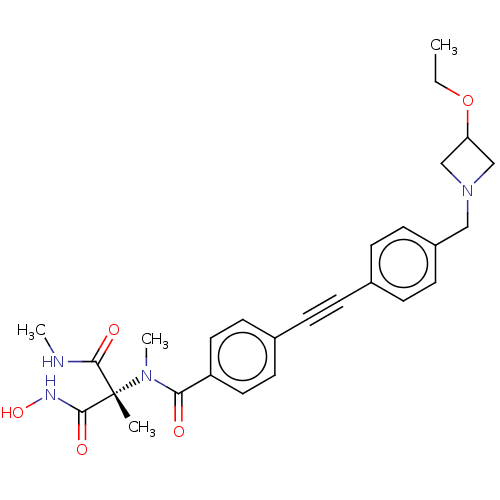
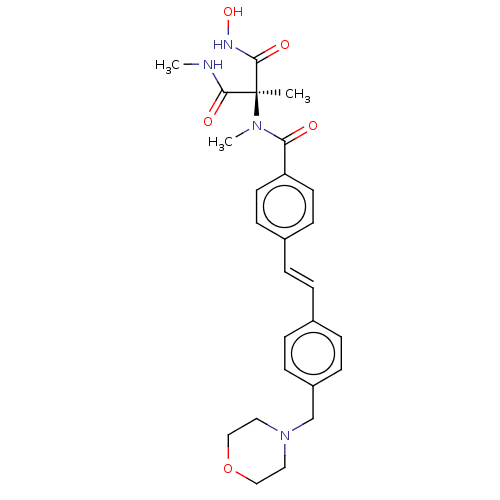
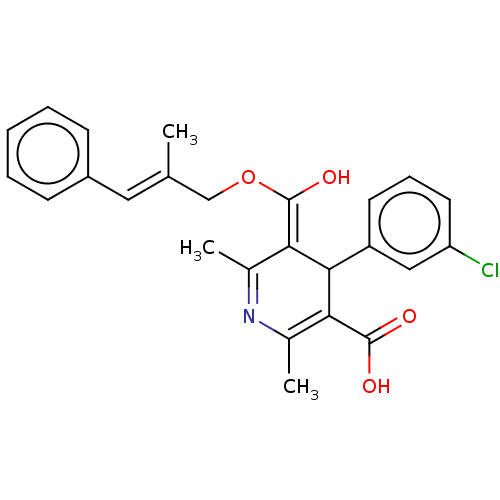
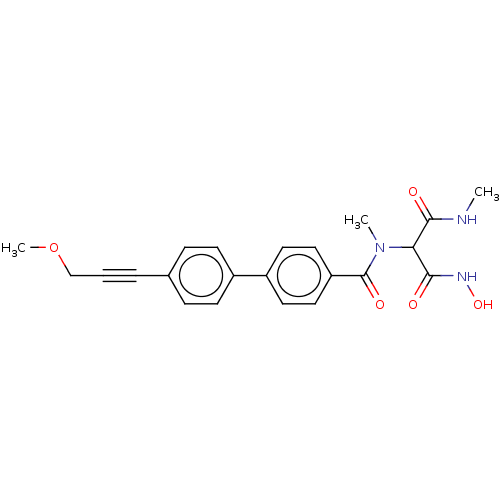
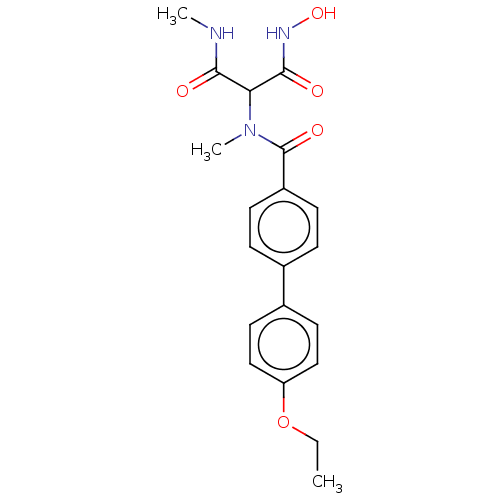
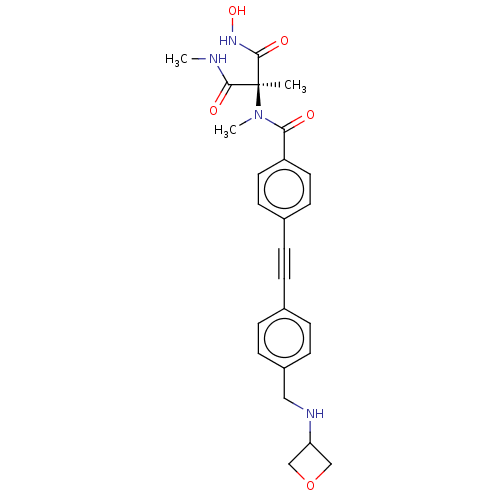
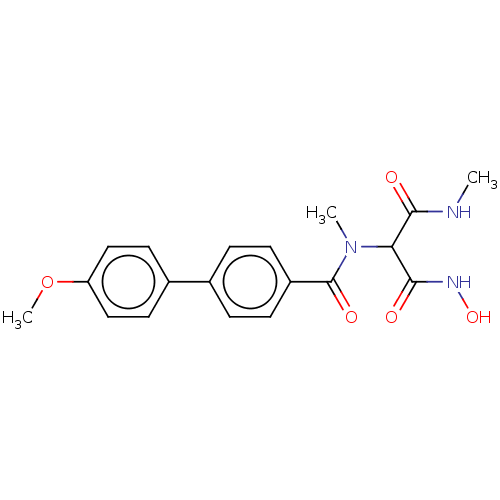
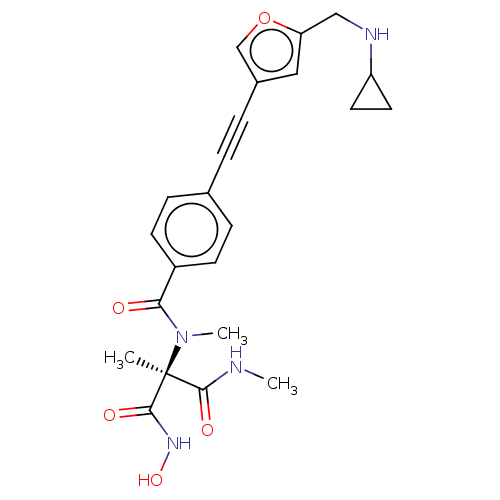
Affinity DataKi: 0.450nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.680nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.30E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 4.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 7.10E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 8.00E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1S(Rattus norvegicus)
Ajinomoto
Curated by ChEMBL
Ajinomoto
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus methodMore data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1S(Rattus norvegicus)
Ajinomoto
Curated by ChEMBL
Ajinomoto
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of Voltage-dependent L-type calcium channel in rat thoracic aorta ringMore data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1S(Rattus norvegicus)
Ajinomoto
Curated by ChEMBL
Ajinomoto
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of voltage-dependent L-type calcium channel in rat thoracic aorta ring assessed as effect on high K+ induced contraction by Magnus methodMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Pseudomonas aeruginosa)
Taisho Pharmaceutical
US Patent
Taisho Pharmaceutical
US Patent
Affinity DataIC50: 1.60nMpH: 8.0 T: 2°CAssay Description:To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an...More data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Pseudomonas aeruginosa)
Taisho Pharmaceutical
US Patent
Taisho Pharmaceutical
US Patent
Affinity DataIC50: 2nMpH: 8.0 T: 2°CAssay Description:To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an...More data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Pseudomonas aeruginosa)
Taisho Pharmaceutical
US Patent
Taisho Pharmaceutical
US Patent
Affinity DataIC50: 2.30nMpH: 8.0 T: 2°CAssay Description:To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an...More data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Pseudomonas aeruginosa)
Taisho Pharmaceutical
US Patent
Taisho Pharmaceutical
US Patent
Affinity DataIC50: 2.80nMpH: 8.0 T: 2°CAssay Description:To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an...More data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1S(Rattus norvegicus)
Ajinomoto
Curated by ChEMBL
Ajinomoto
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus methodMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Pseudomonas aeruginosa)
Taisho Pharmaceutical
US Patent
Taisho Pharmaceutical
US Patent
Affinity DataIC50: 2.90nMpH: 8.0 T: 2°CAssay Description:To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an...More data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Pseudomonas aeruginosa)
Taisho Pharmaceutical
US Patent
Taisho Pharmaceutical
US Patent
Affinity DataIC50: 3.10nMpH: 8.0 T: 2°CAssay Description:To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an...More data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Pseudomonas aeruginosa)
Taisho Pharmaceutical
US Patent
Taisho Pharmaceutical
US Patent
Affinity DataIC50: 3.10nMpH: 8.0 T: 2°CAssay Description:To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an...More data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Pseudomonas aeruginosa)
Taisho Pharmaceutical
US Patent
Taisho Pharmaceutical
US Patent
Affinity DataIC50: 3.30nMpH: 8.0 T: 2°CAssay Description:To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an...More data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Pseudomonas aeruginosa)
Taisho Pharmaceutical
US Patent
Taisho Pharmaceutical
US Patent
Affinity DataIC50: 3.40nMpH: 8.0 T: 2°CAssay Description:To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an...More data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1S(Rattus norvegicus)
Ajinomoto
Curated by ChEMBL
Ajinomoto
Curated by ChEMBL
Affinity DataIC50: 3.70nMAssay Description:Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus methodMore data for this Ligand-Target Pair