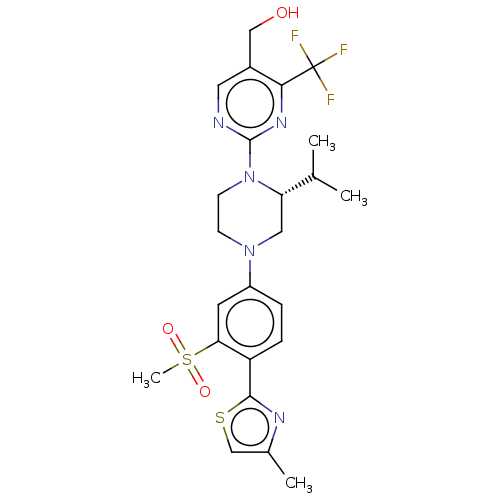
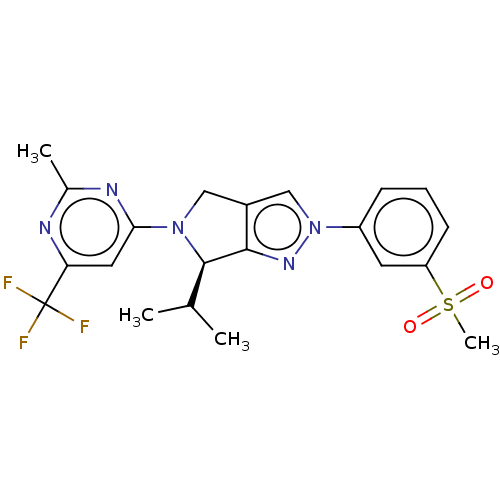
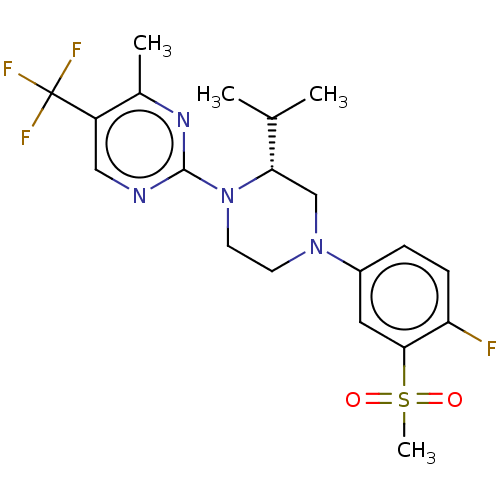
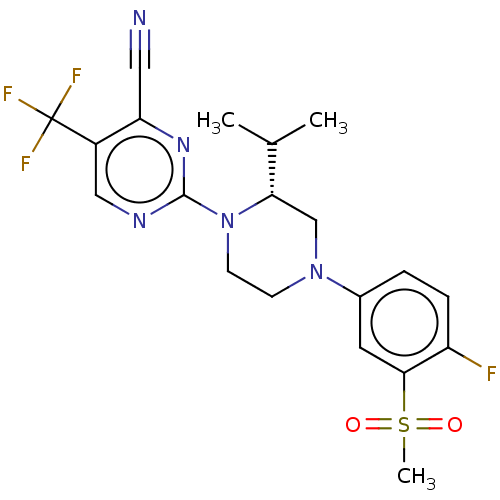
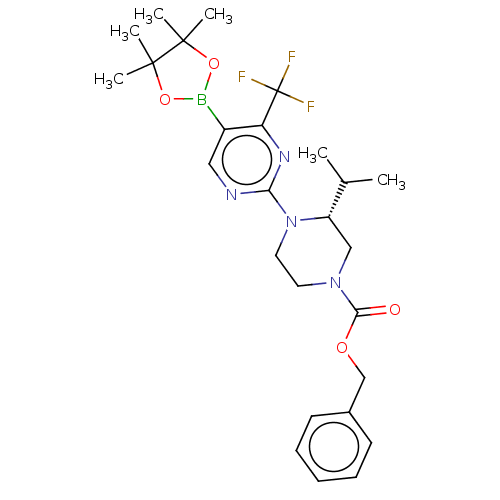
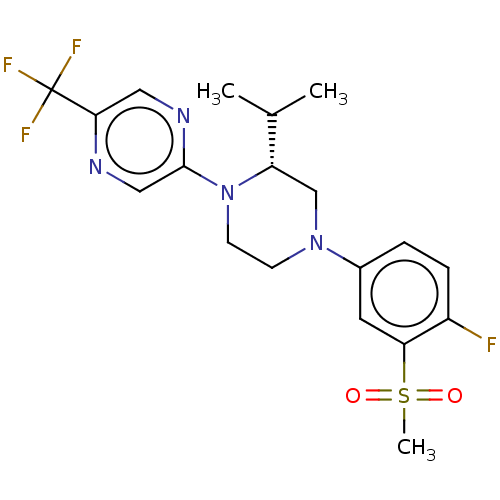
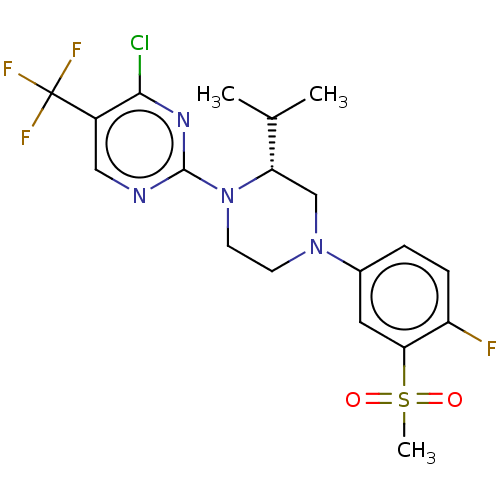
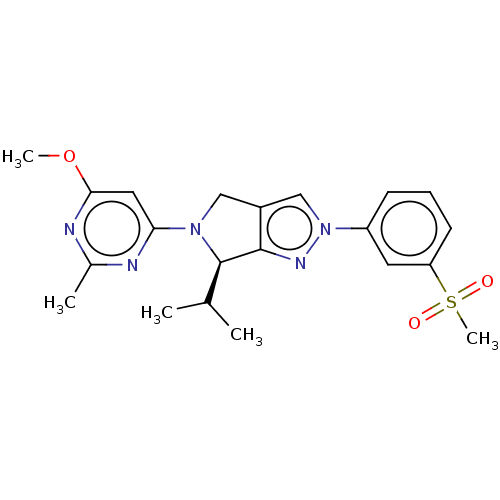
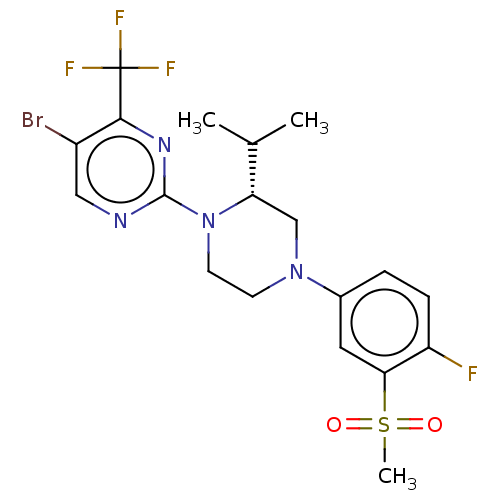
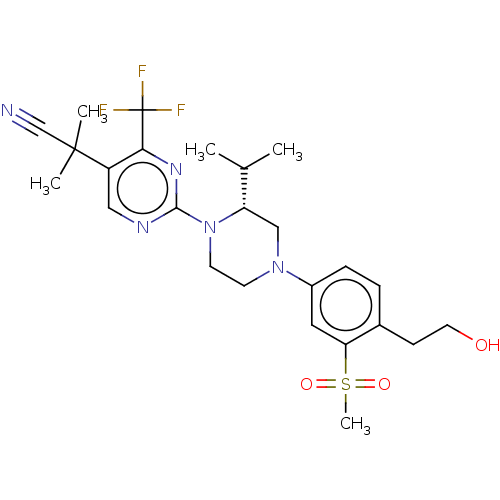
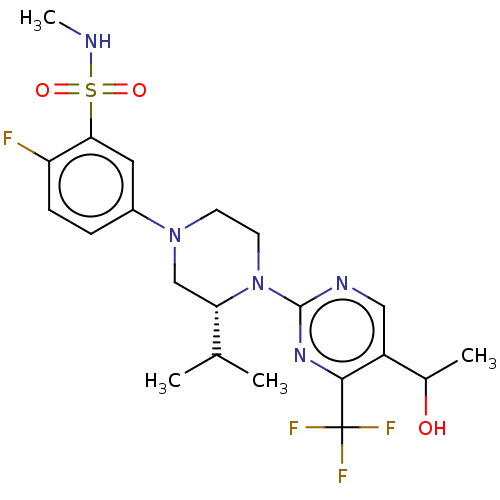
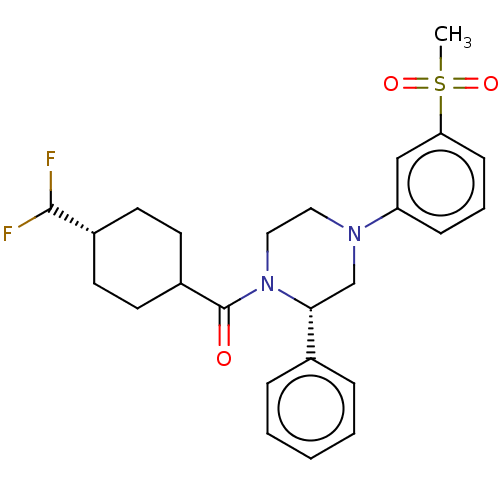
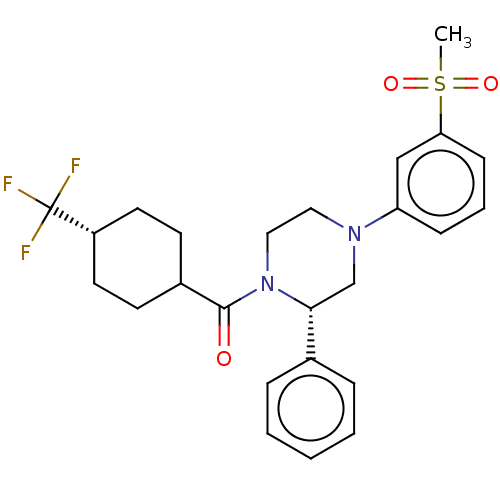
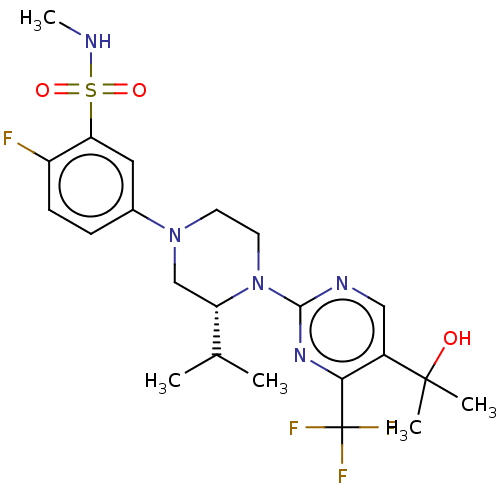
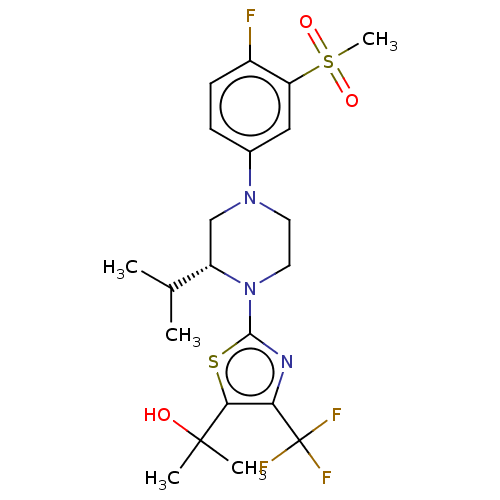
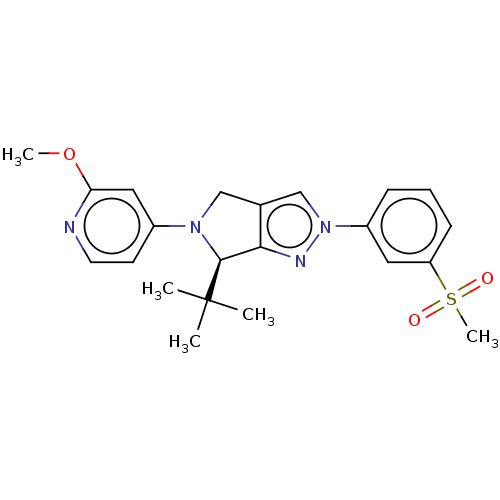
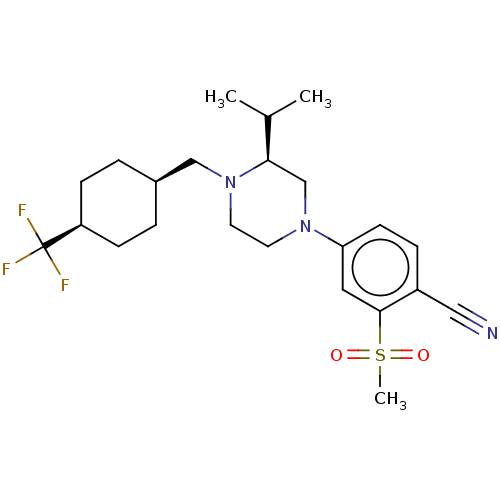
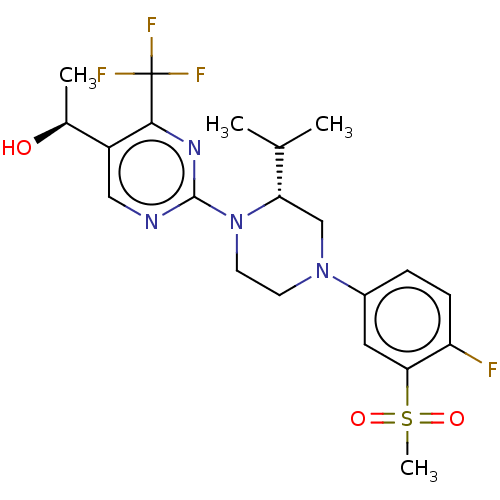
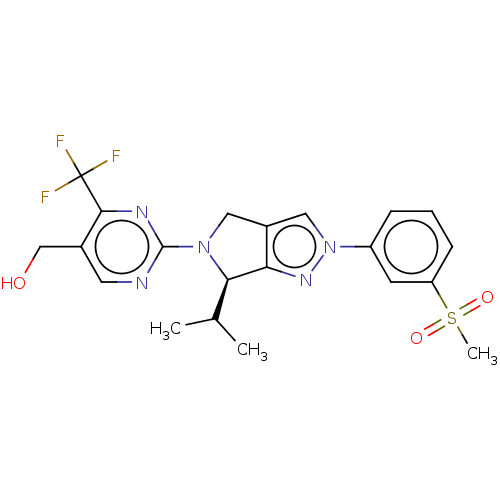
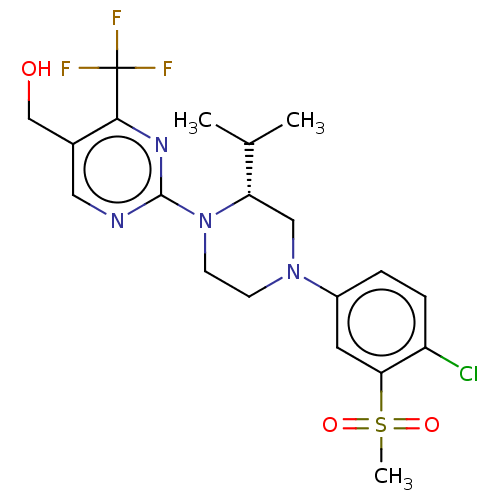
Affinity DataKi: 0.200nMAssay Description:Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from recombinant hum...More data for this Ligand-Target Pair
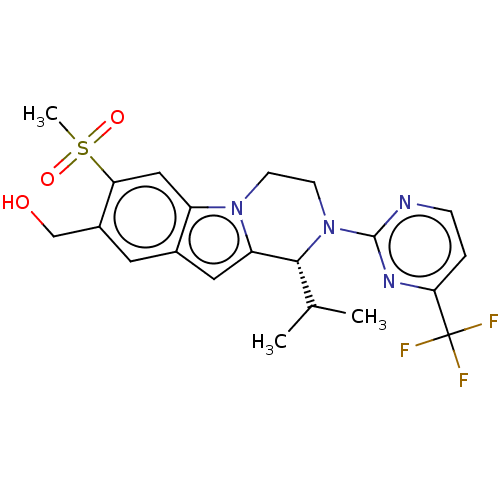
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
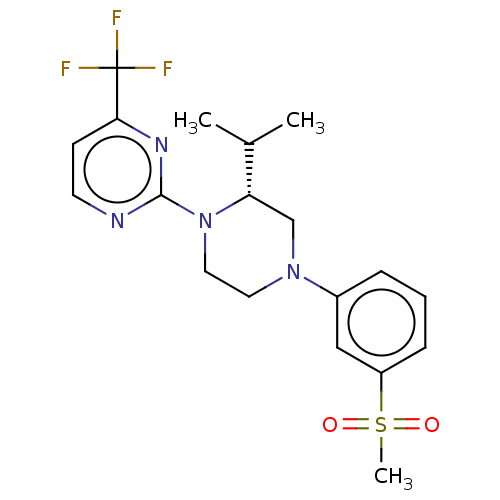
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
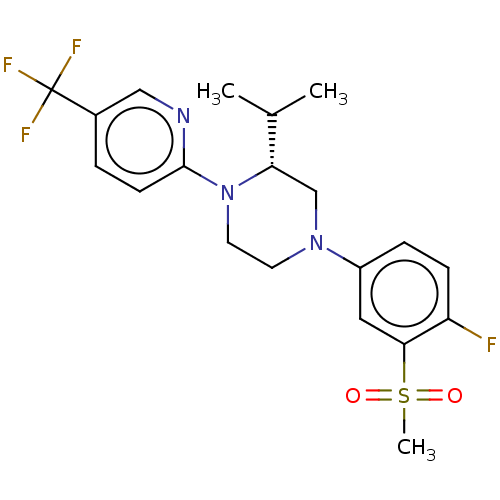
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair

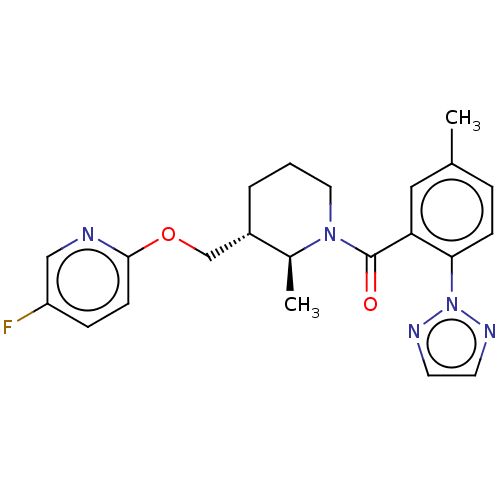
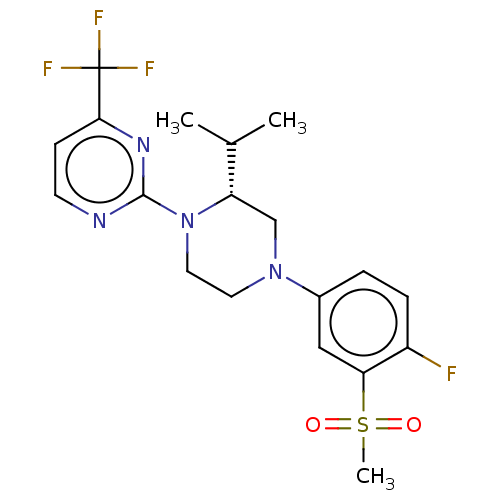
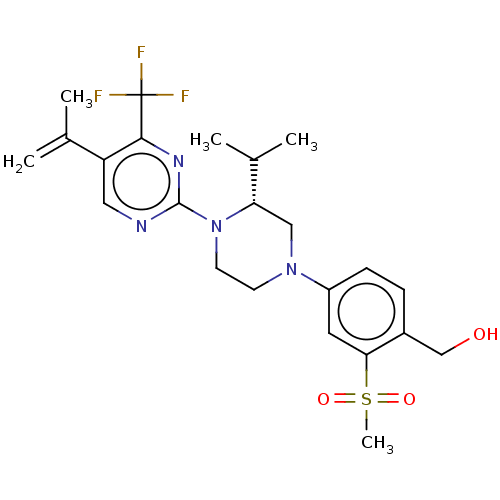
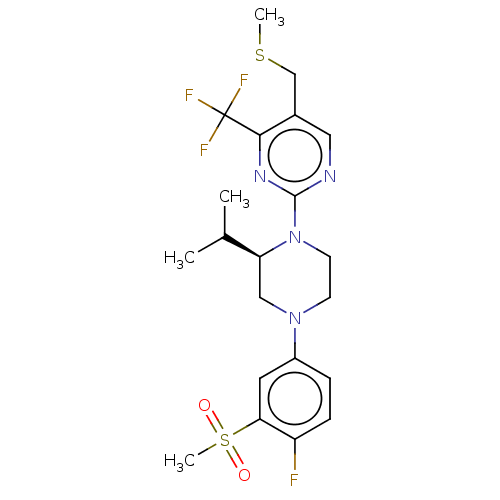
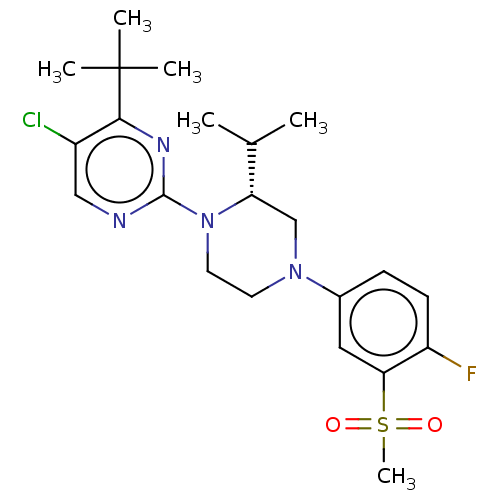
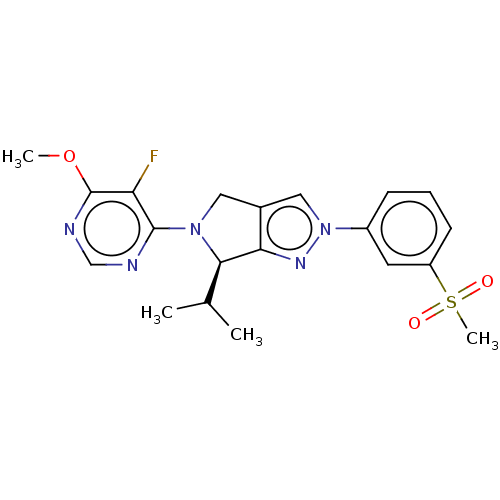
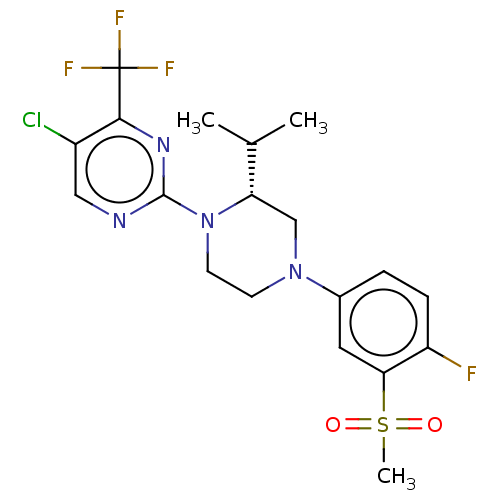
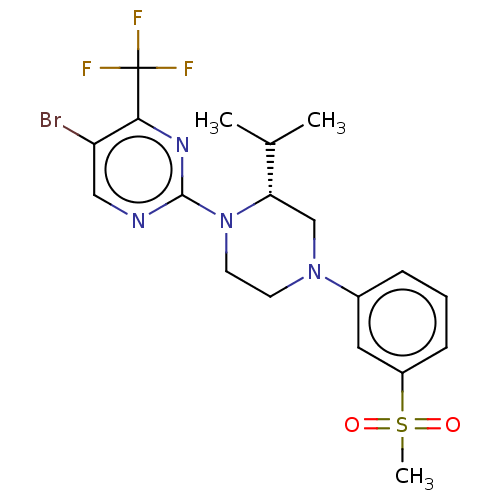
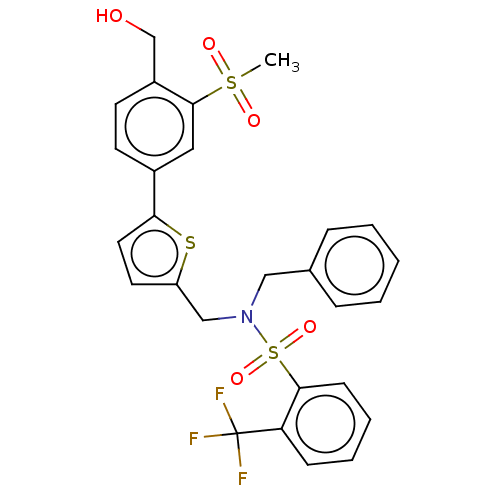

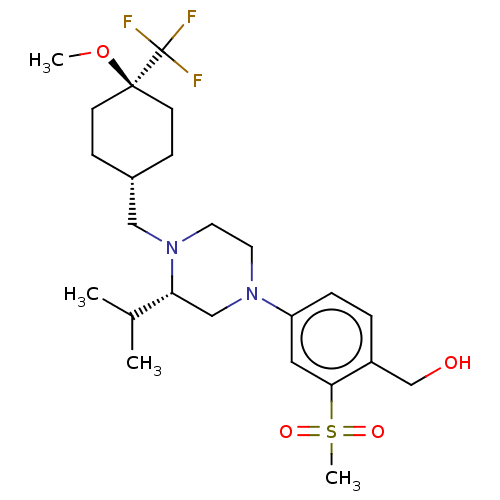
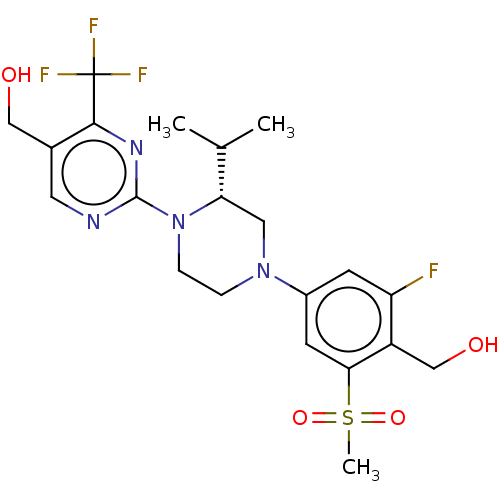
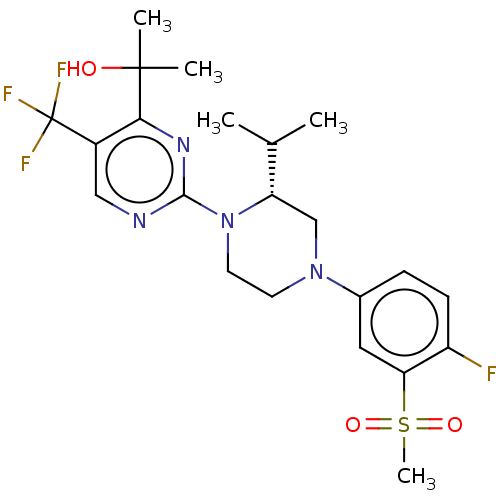
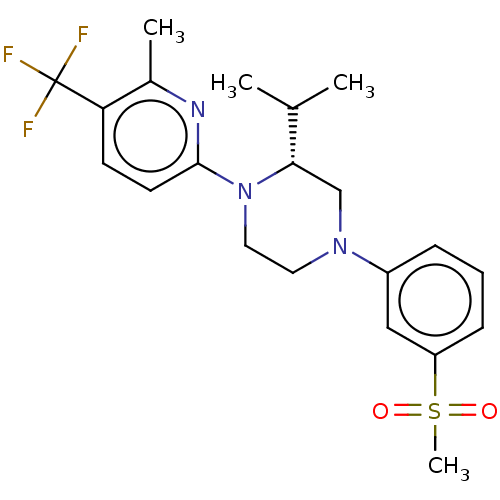
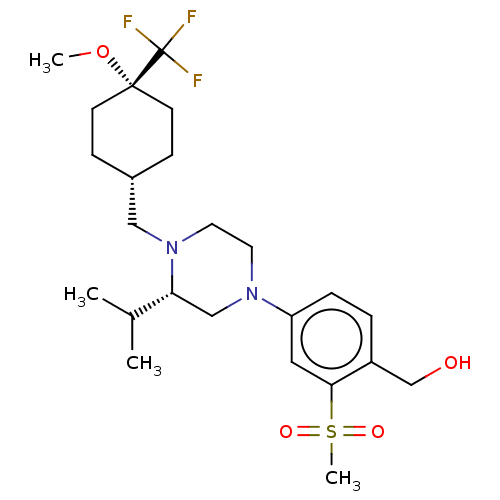
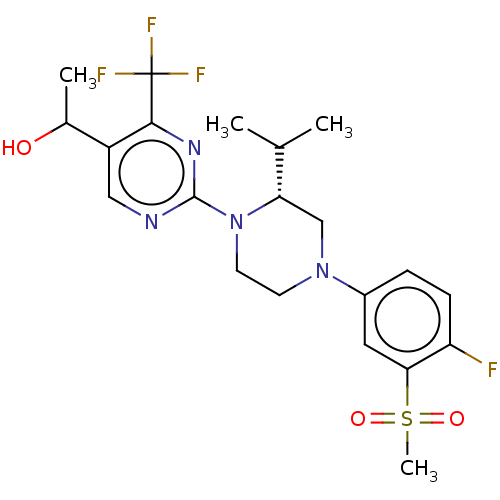
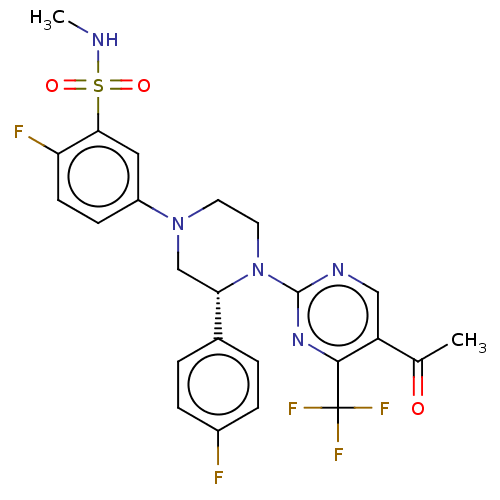
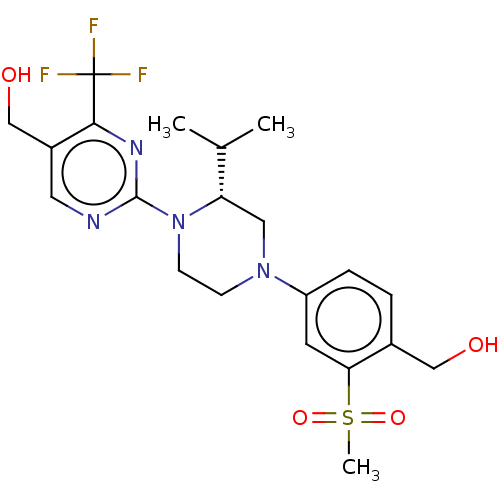
 3D Structure (crystal)
3D Structure (crystal)