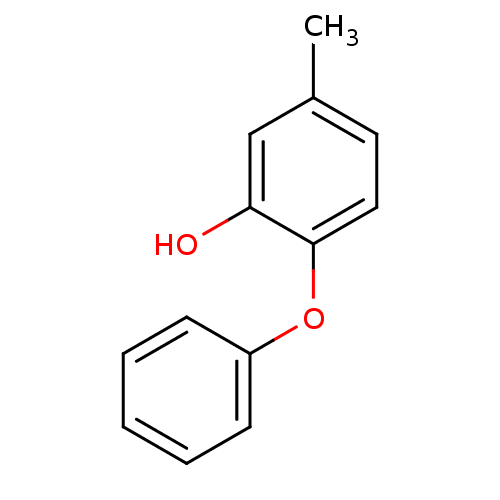
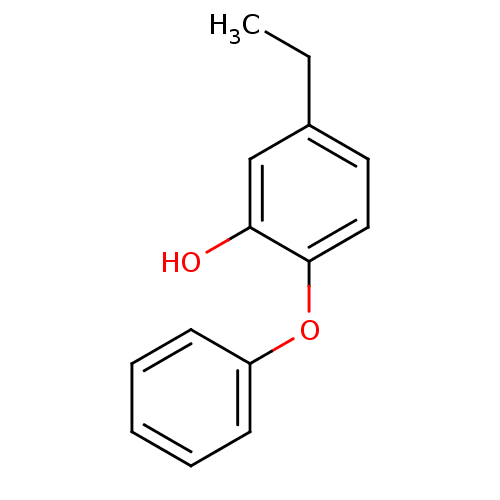
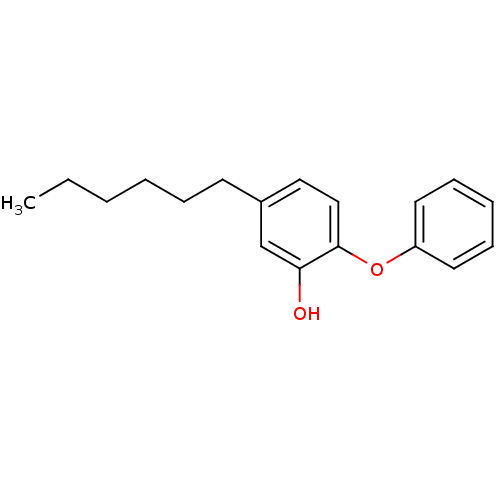
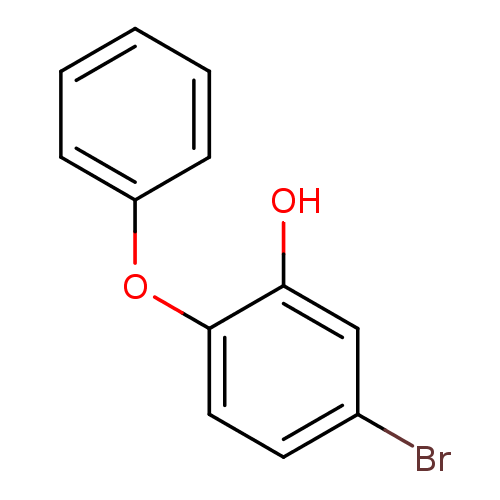
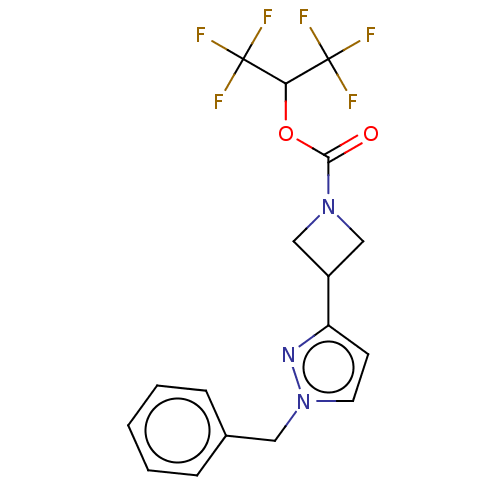
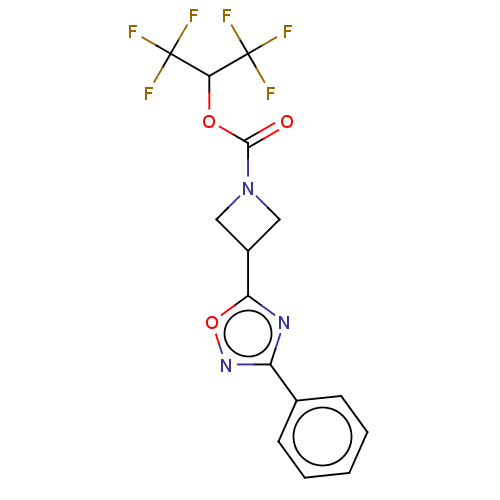
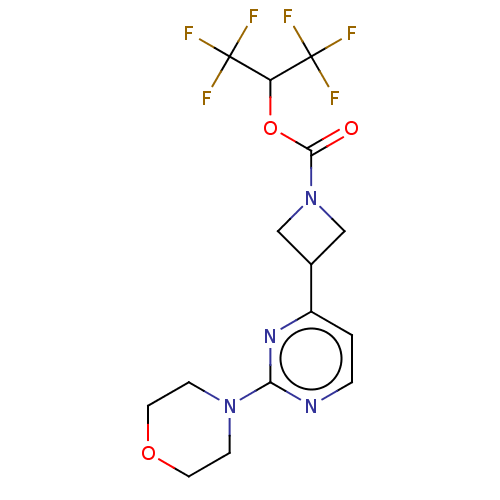
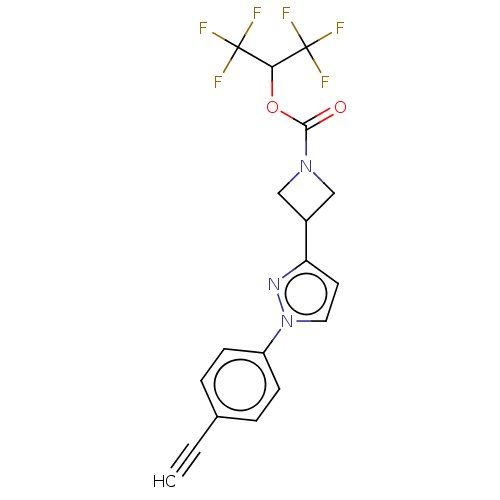
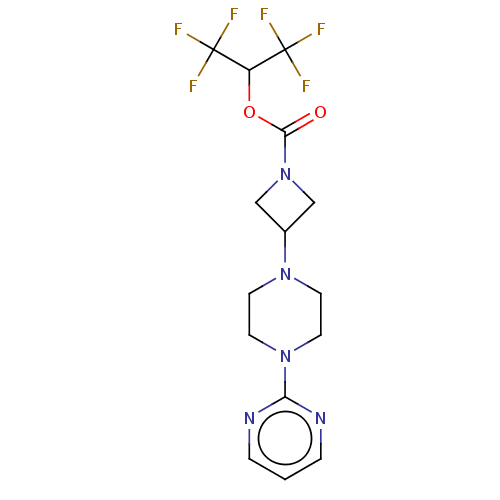
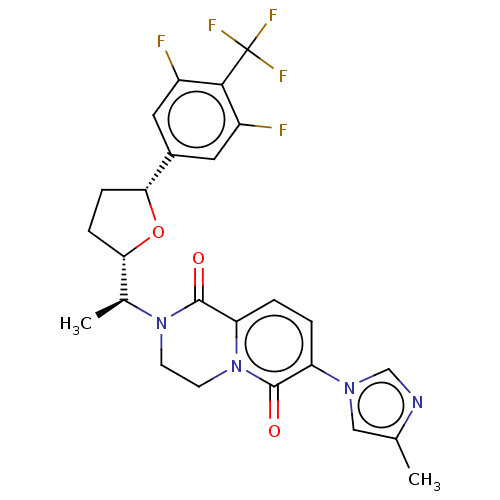
Affinity DataKi: 0.0510nM ΔG°: -58.7kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
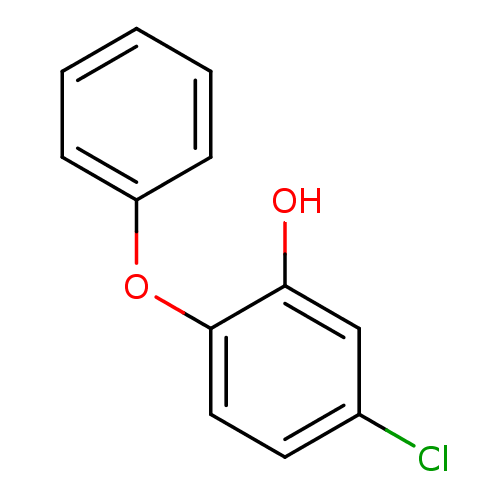
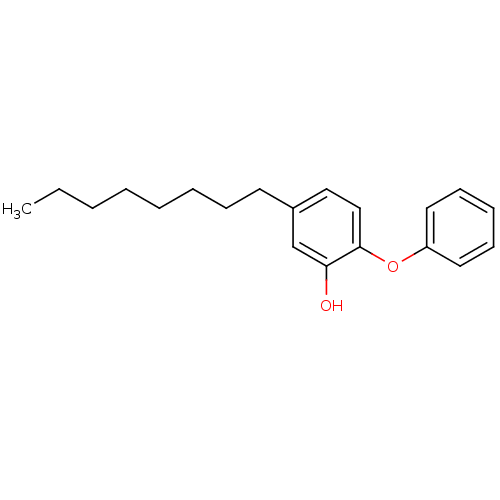
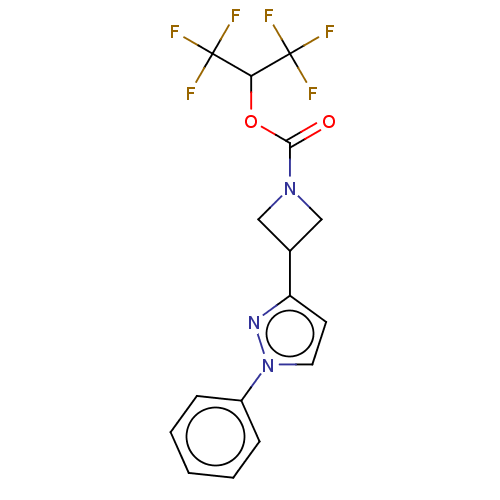
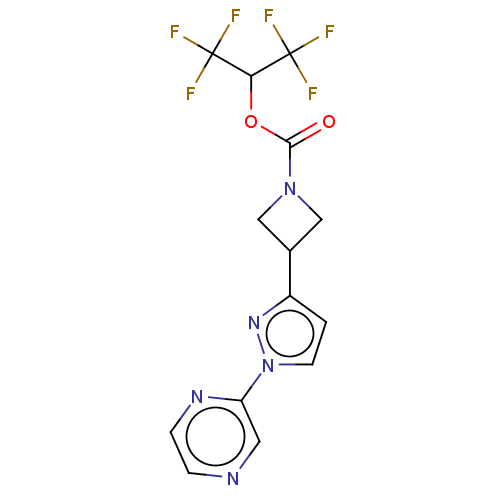
Affinity DataKi: 0.440nM ΔG°: -53.4kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH](Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Stony Brook University
Curated by ChEMBL
Stony Brook University
Curated by ChEMBL
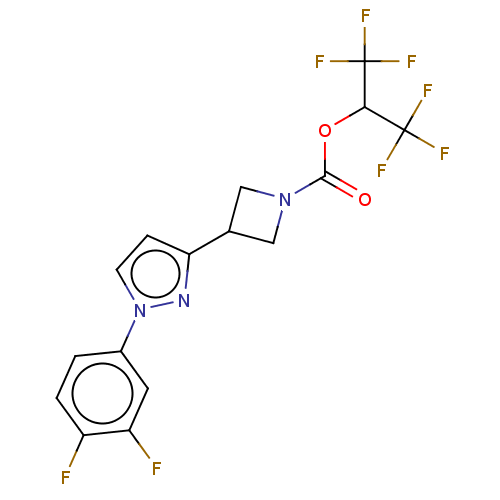
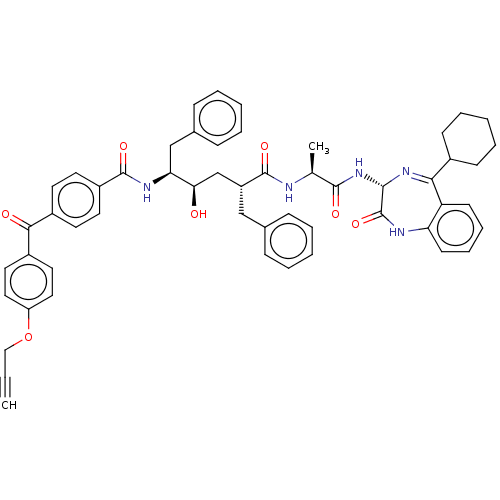
Affinity DataKi: 1nMAssay Description:Inhibition of Mycobacterium tuberculosis H37RV InhAMore data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH](Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Stony Brook University
Curated by ChEMBL
Stony Brook University
Curated by ChEMBL
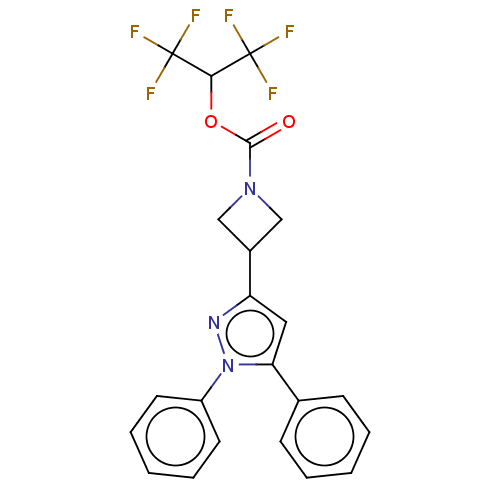
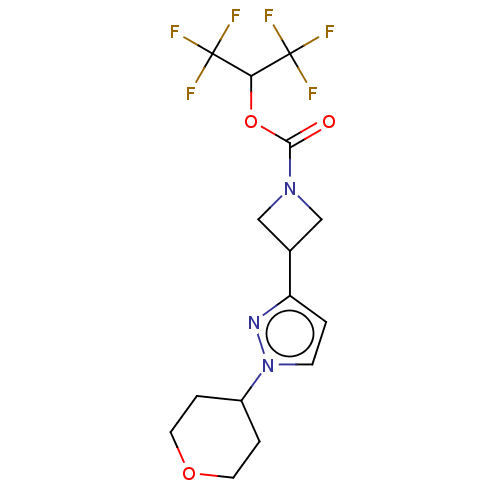
Affinity DataKi: 1nMAssay Description:Inhibition of Mycobacterium tuberculosis H37RV InhAMore data for this Ligand-Target Pair
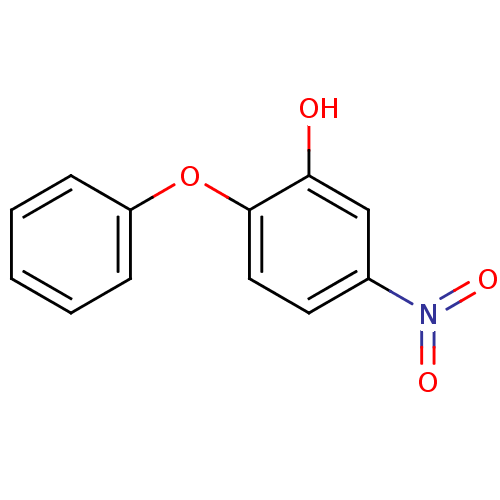
Affinity DataKi: 1.30nM ΔG°: -50.7kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
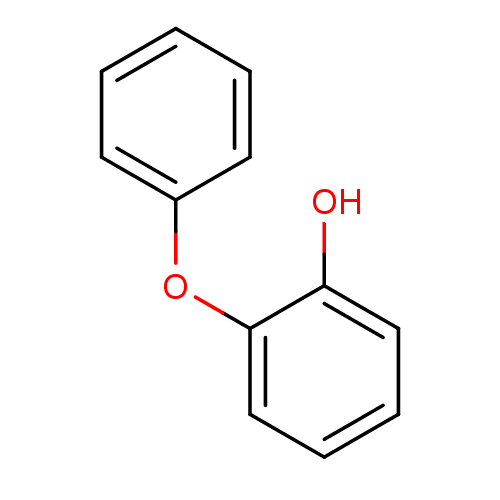
Affinity DataKi: 1.90nM ΔG°: -49.8kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 1.90nM ΔG°: -49.8kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 2.10nM ΔG°: -49.5kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 2.70nM ΔG°: -48.9kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 7.10nM ΔG°: -46.5kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 23.6nM ΔG°: -43.5kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 149nM ΔG°: -39.0kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
Affinity DataKi: 192nM ΔG°: -38.3kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH](Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Stony Brook University
Curated by ChEMBL
Stony Brook University
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Inhibition of Mycobacterium tuberculosis H37RV InhAMore data for this Ligand-Target Pair
Affinity DataKi: 247nM ΔG°: -37.7kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
Affinity DataKi: 289nM ΔG°: -37.3kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 308nM ΔG°: -37.2kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
Affinity DataKi: 311nM ΔG°: -37.1kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
Affinity DataKi: 331nM ΔG°: -37.0kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
Affinity DataKi: 696nM ΔG°: -35.1kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
Affinity DataKi: 789nM ΔG°: -34.8kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 2.26E+3nM ΔG°: -32.2kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nM ΔG°: -30.3kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.410nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.490nMAssay Description:Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMAssay Description:Inhibition of gamma-secretase (unknown origin) using Notch1 substrate assessed as Notch1-NICD productionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMAssay Description:Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of gamma-secretase (unknown origin) using APP substrate assessed as amyloid-beta40 productionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.740nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.910nMAssay Description:Inhibition of gamma-secretase (unknown origin) using APP substrate assessed as amyloid-beta40 productionMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of gamma-secretase (unknown origin) using Notch1 substrate assessed as Notch1-NICD productionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 3.34nMT: 2°CAssay Description:The ability of compounds to modulate production of amyloid beta protein
Abeta(1-42) was determined using human WT-APP overexpressing CHO cells.
Cel...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of gamma-secretase in cell-free human HeLa membrane assessed as amyloid beta-42 production using APP as substrateMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B(Homo sapiens (Human))
Pfizer
Curated by ChEMBL
Pfizer
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of full length recombinant human PDE1B1 using 3',5'-[3H]cAMP as substrate after 30 mins by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.48nMT: 2°CAssay Description:The ability of compounds to modulate production of amyloid beta protein
Abeta(1-42) was determined using human WT-APP overexpressing CHO cells.
Cel...More data for this Ligand-Target Pair
Affinity DataIC50: 5.78nMT: 2°CAssay Description:The ability of compounds to modulate production of amyloid beta protein
Abeta(1-42) was determined using human WT-APP overexpressing CHO cells.
Cel...More data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Pfizer
Curated by ChEMBL
Pfizer
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Modulation of human gamma secretase overexpressed in CHO cells co-expressing wild type human APP assessed as inhibition of amyloid beta-42 production...More data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Pfizer
Curated by ChEMBL
Pfizer
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of gamma-secretase (unknown origin) expressed in CHO cells overexpressing human wild type APP assessed as reduction of amyloid beta 42 lev...More data for this Ligand-Target Pair
Affinity DataIC50: 7.84nMT: 2°CAssay Description:The ability of compounds to modulate production of amyloid beta protein
Abeta(1-42) was determined using human WT-APP overexpressing CHO cells.
Cel...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)