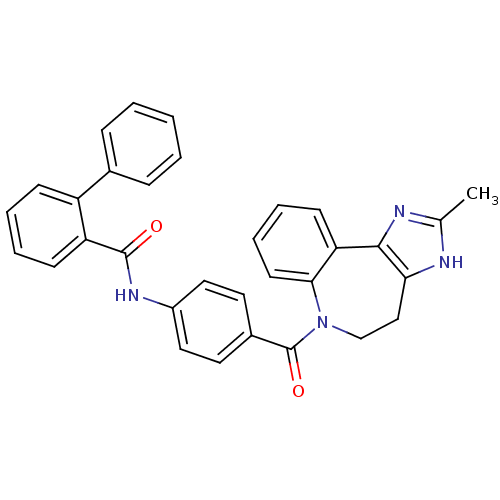
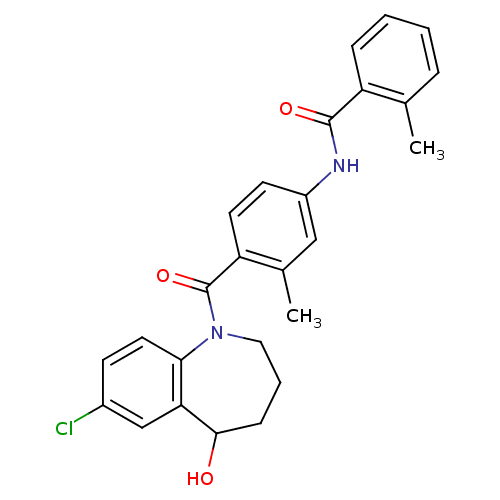
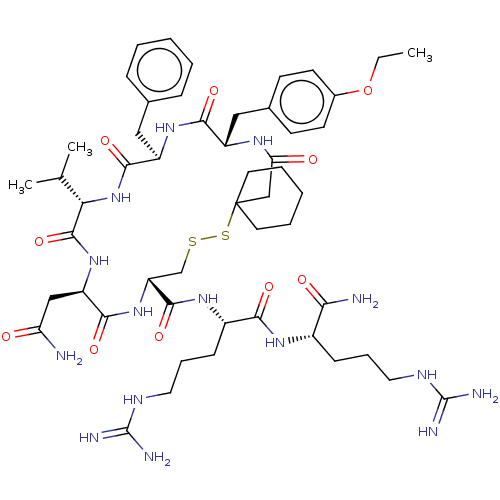
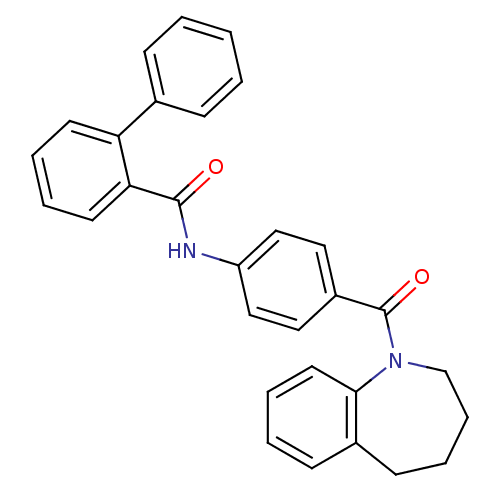
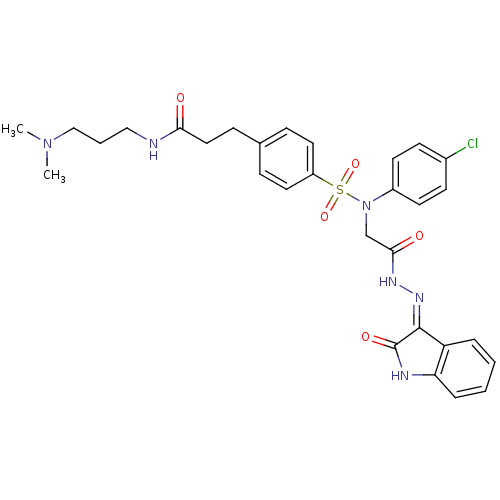
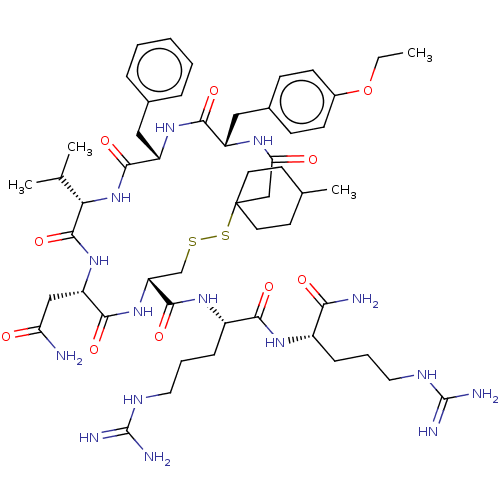
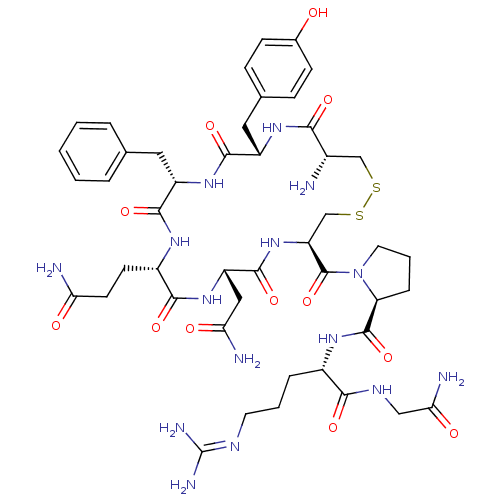
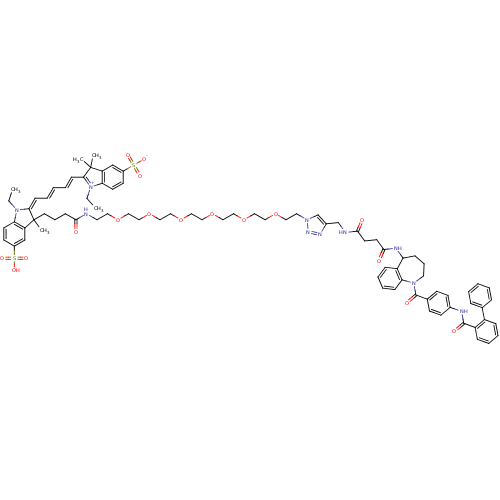
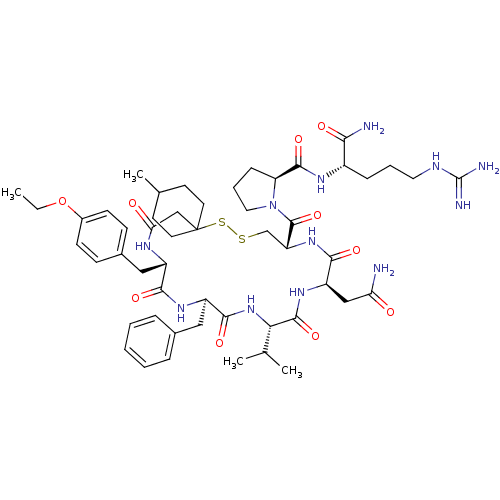
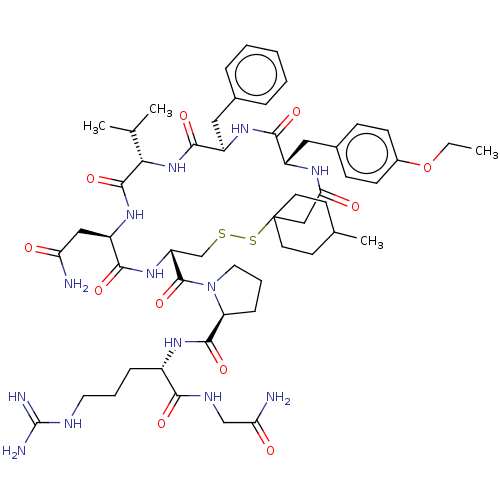
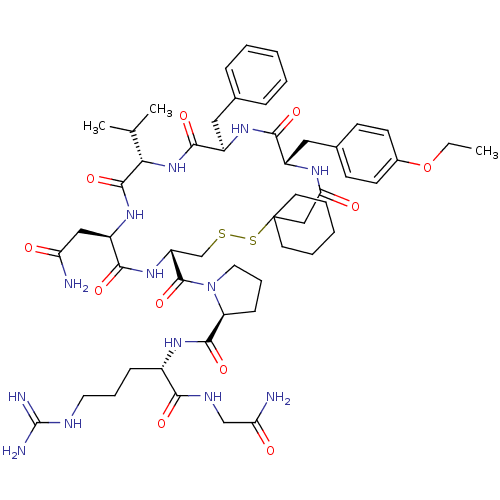
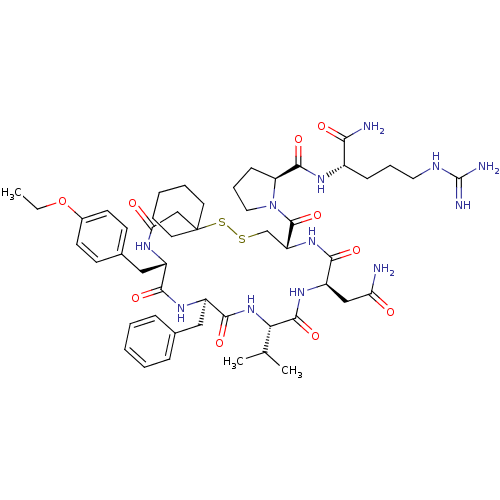
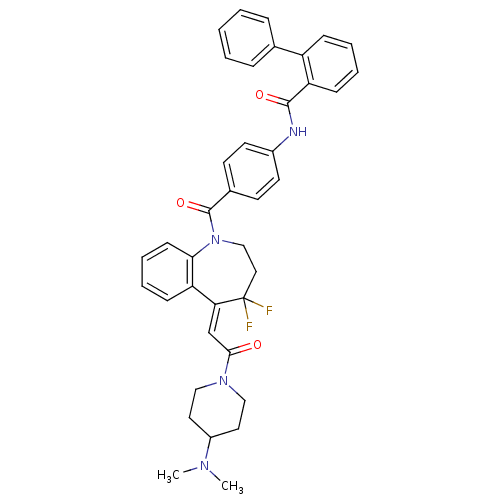
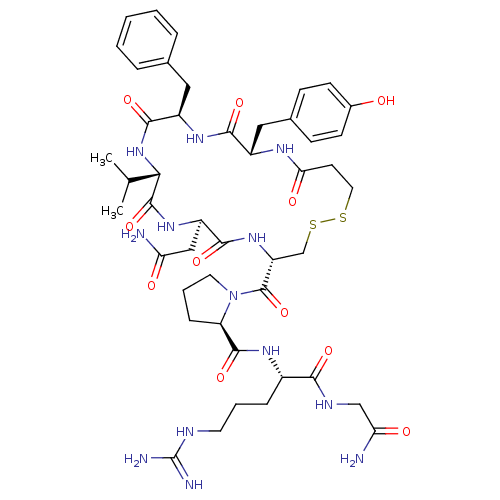
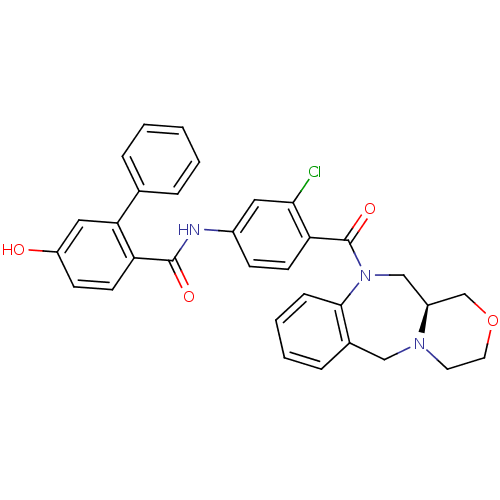
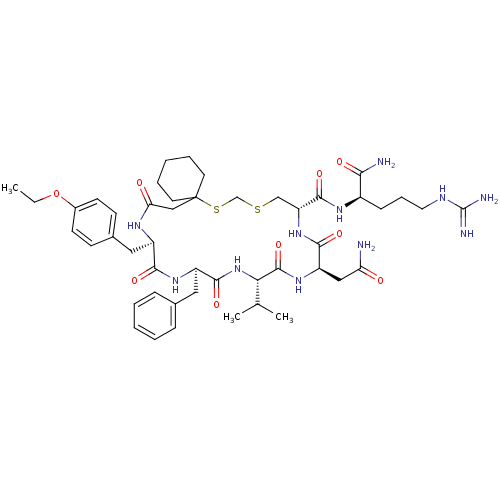
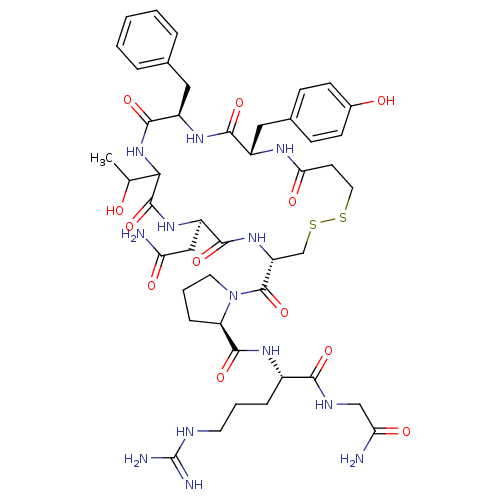
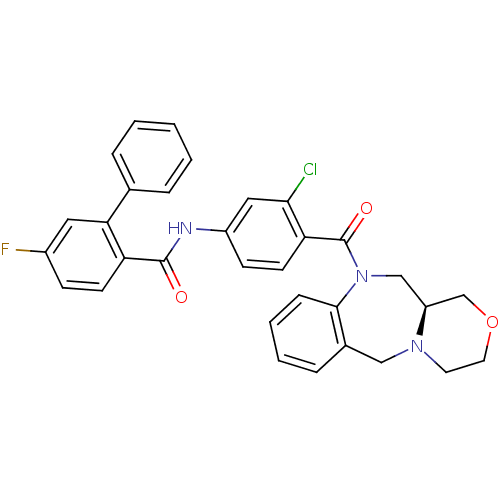
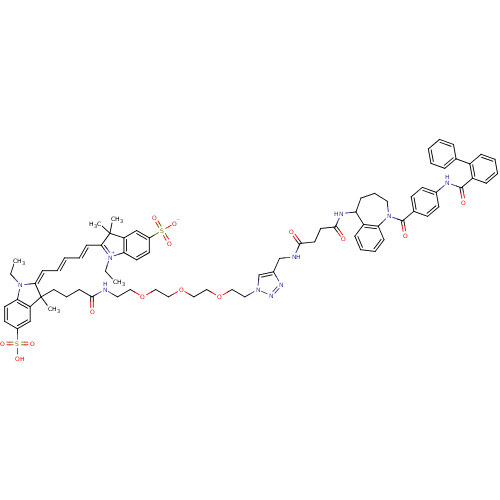
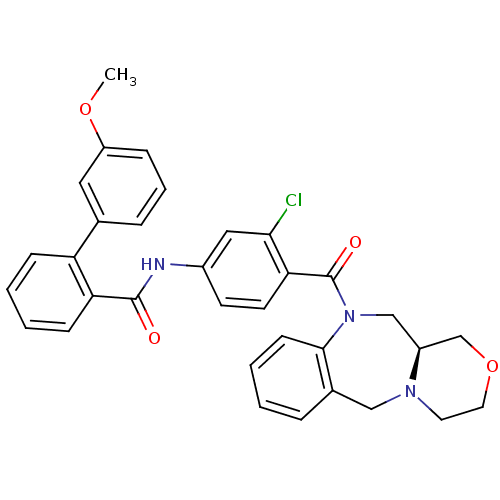
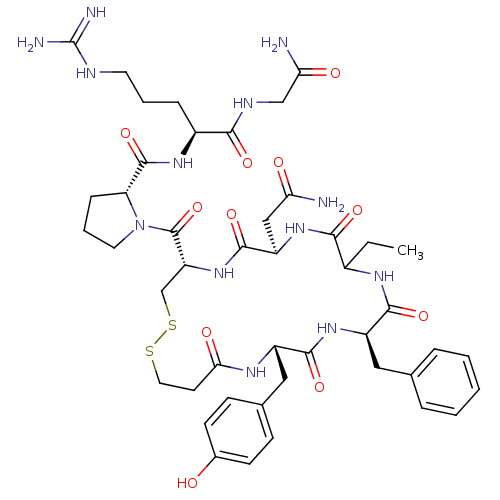
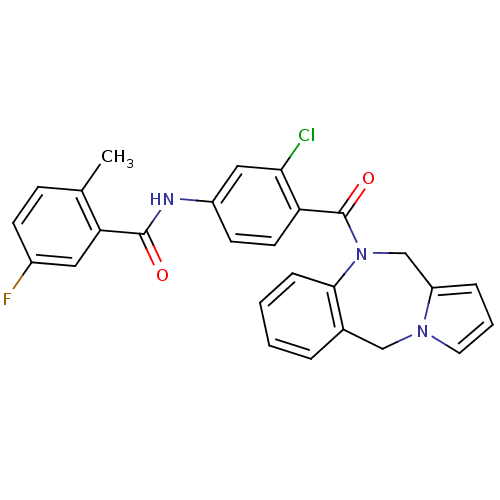
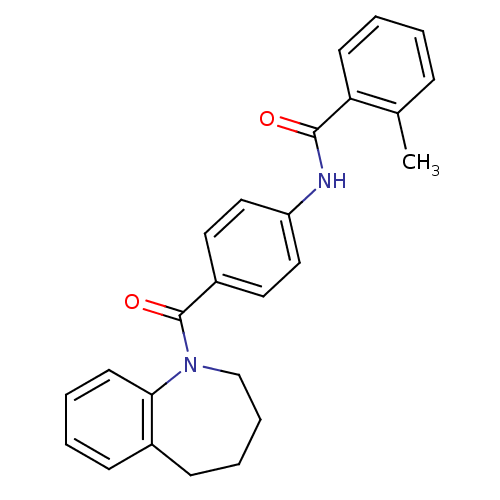
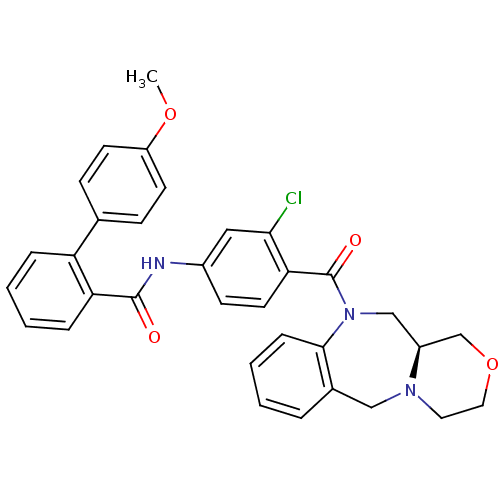
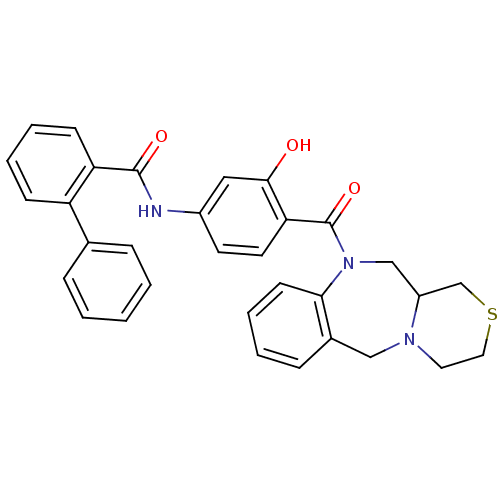
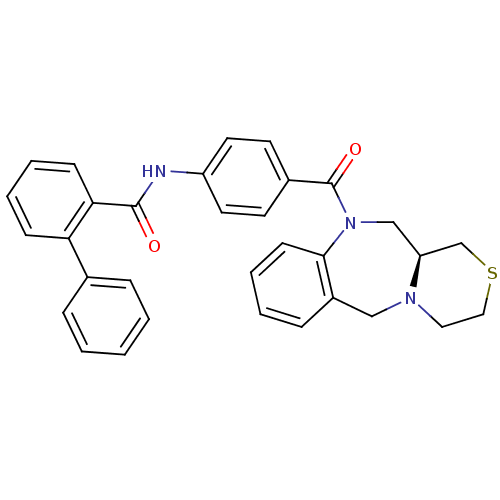
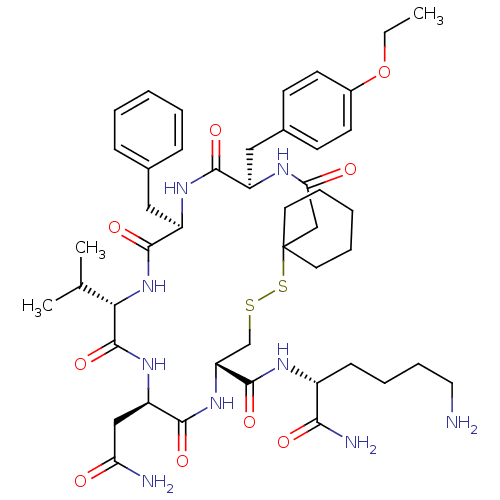
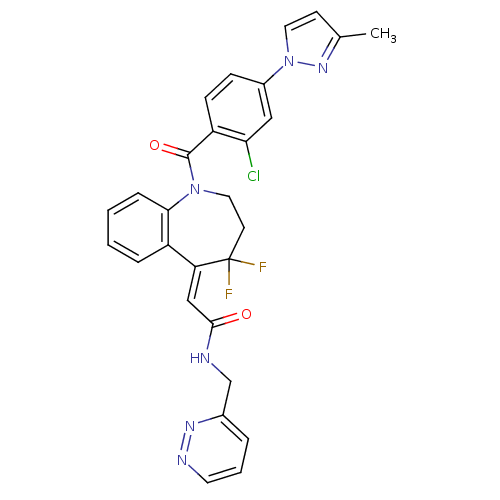
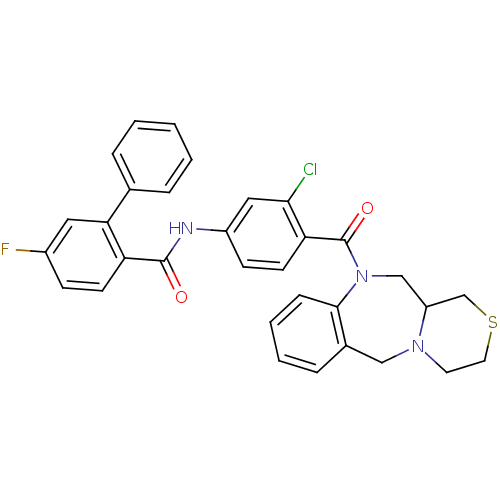
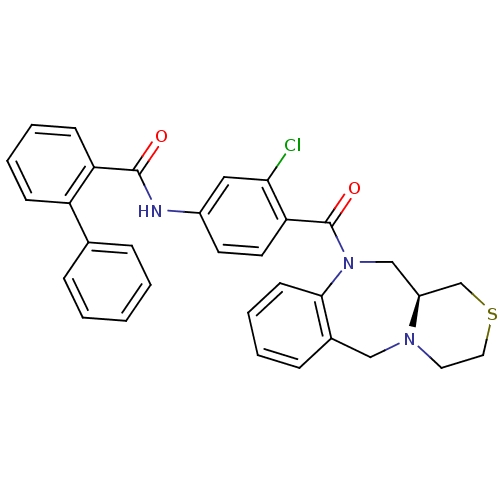
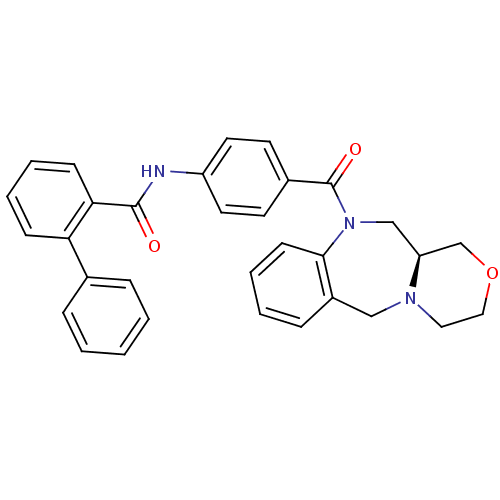
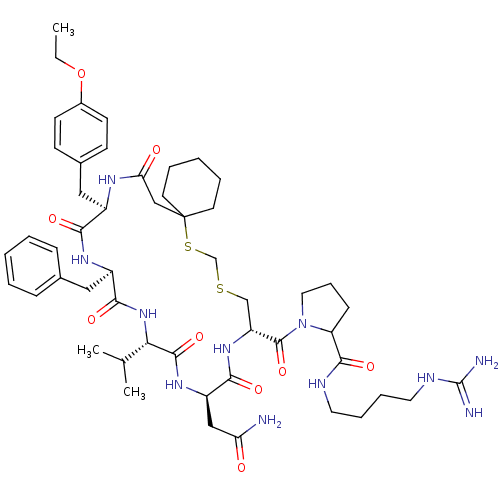
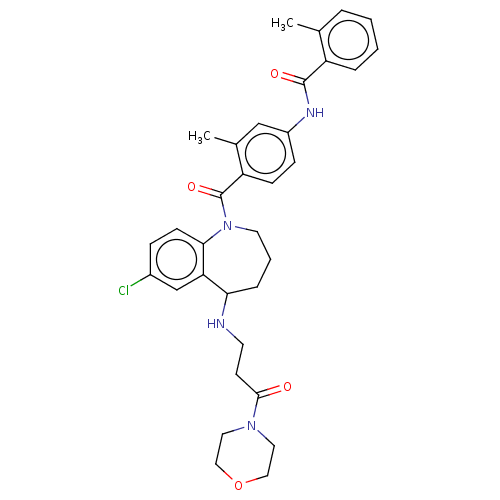
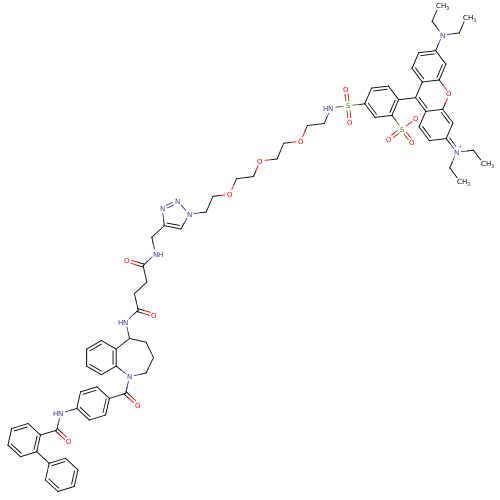
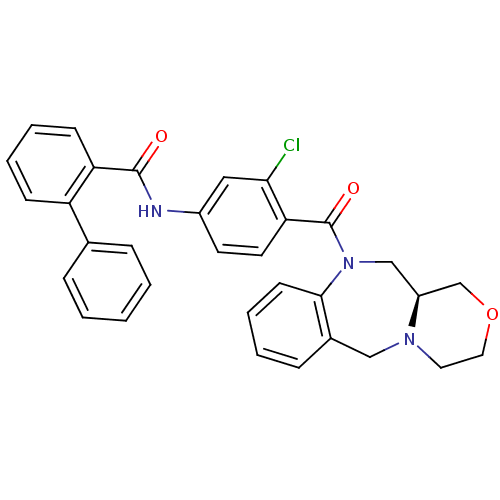
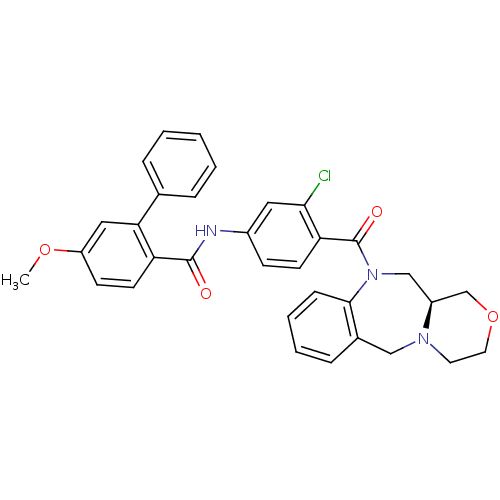
Affinity DataKi: 0.360nMAssay Description:Displacement [3H]Arg human recombinant Vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50%More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation.More data for this Ligand-Target Pair
Affinity DataKi: 0.570nMAssay Description:Displacement of [3H]-AVP from human V2 receptor expressed in CHO cell membranes by radioligand binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.570nMAssay Description:Displacement of [3H]AVP from human vasopressin V2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.570nMAssay Description:Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 minsMore data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:Displacement of [3H]-vasopressin from vasopressin V2 receptor in human kidney tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation.More data for this Ligand-Target Pair
Affinity DataKi: 0.830nMAssay Description:Displacement of [3H]-AVP from SNAP-tagged vasopressin V2 receptor expressed in HEK293 cells by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.990nMAssay Description:Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation.More data for this Ligand-Target Pair
Affinity DataKi: 0.990nMAssay Description:Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation.More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation.More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]-arginine-vasopressin from human V2 receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindin...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation.More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation.More data for this Ligand-Target Pair
Affinity DataKi: 1.19nMAssay Description:Binding affinity at cloned human Vasopressin V2 receptor stably expressed in CHO cells, using [3H]-AVP as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Binding affinity against human vasopressin V2 receptor was determined by using plasma membranes from CHO cells stably transfected with VP/OT receptor...More data for this Ligand-Target Pair
Affinity DataKi: 1.36nMAssay Description:Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins by saturation binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.48nMAssay Description:Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 minsMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition constant for vasopressin-stimulated adenylate cyclase (Vasopressin V2 receptor) of medullary membranes of human kidneyMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Binding affinity against human vasopressin V2 receptor was determined by using plasma membranes from CHO cells stably transfected with VP/OT receptor...More data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Displacement of conivaptan-red from SNAP-tagged V2 receptor (unknown origin) expressed in HEK293 cells assessed as inhibition constant measured after...More data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.93nMAssay Description:Displacement of [3H]-AVP from SNAP-tagged vasopressin V2 receptor expressed in HEK293 cells by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Binding affinity against human vasopressin V2 receptor was determined by using plasma membranes from CHO cells stably transfected with VP/OT receptor...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Displacement of conivaptan-red from SNAP-tagged human V2 receptor expressed in HEK293 cells measured after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.68nMAssay Description:Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V1a receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidneyMore data for this Ligand-Target Pair
Affinity DataKi: 3.40nM ΔG°: -11.5kcal/molepH: 7.4 T: 2°CAssay Description:The affinities of test compounds for human V2 receptor were evaluated by the radioligand binding study using membrane fractions isolated from CHO cel...More data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidneyMore data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Displacement of conivaptan-red from SNAP-tagged V2 receptor (unknown origin) expressed in HEK293 cells assessed as inhibition constant measured after...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 minsMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair