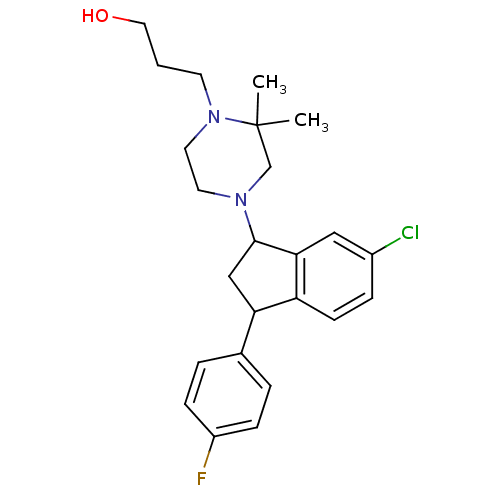
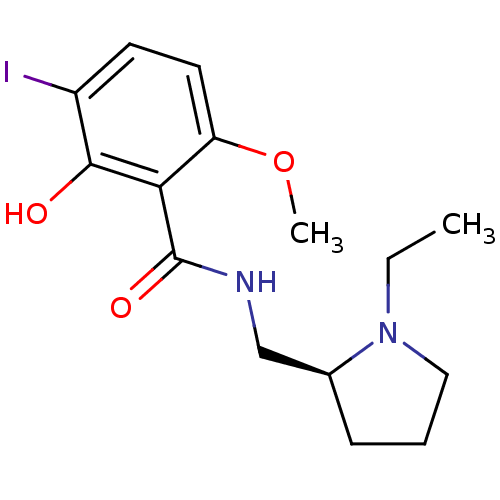
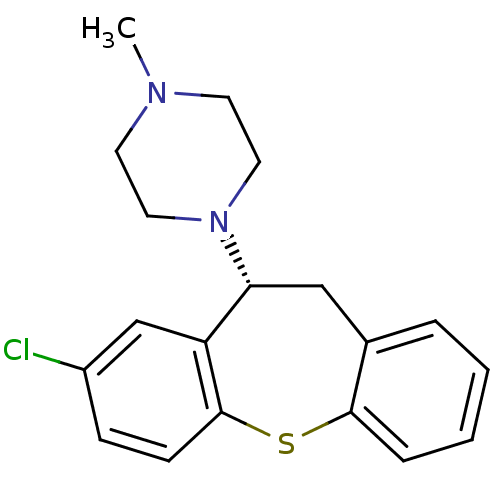
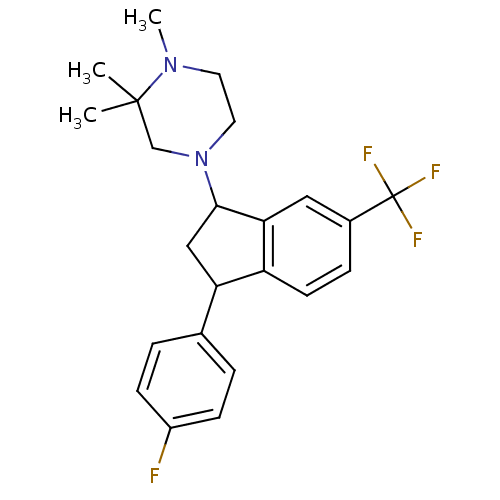
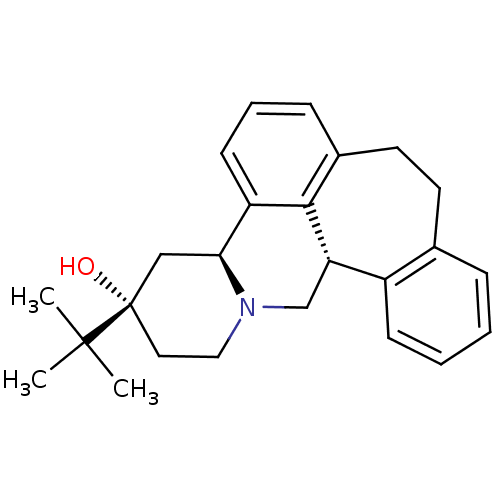
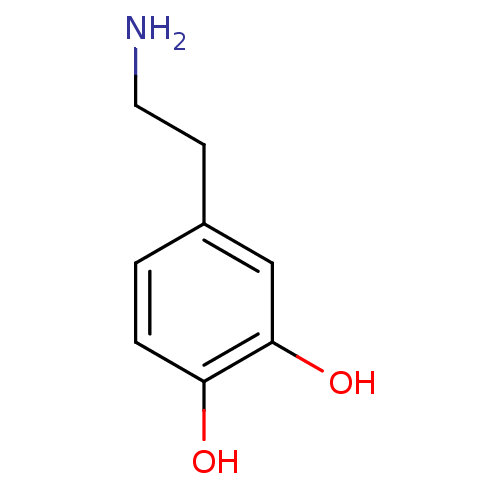
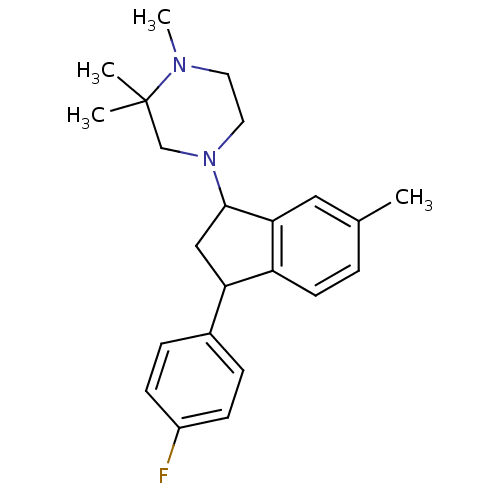
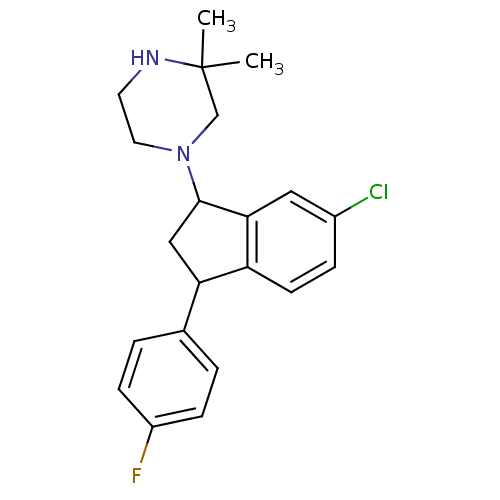
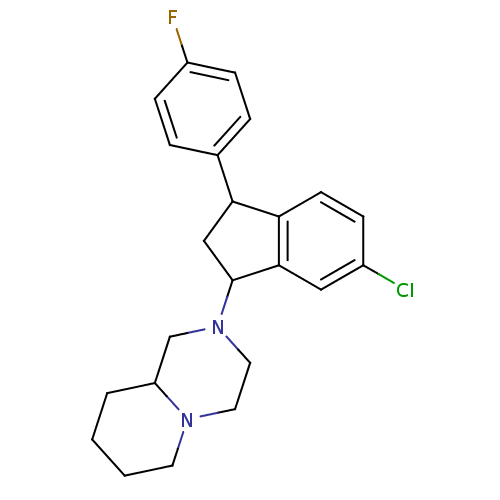
Affinity DataIC50: 0.280nMAssay Description:Ability to inhibit [3H]haloperidol binding to dopamine receptor in rat striatal homogenateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:In vivo binding affinity of the compound against dopamine (D1) receptor in rat caudate-putamen tissue using [3H]-SCH-23,390 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:In vitro affinity to dopamine receptor using [3H]NPAD as radioligand in rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Binding affinity against dopamine receptor in rat striatal membrane using [3H]haloperidolMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 0.570nMAssay Description:Binding affinity against Dopamine receptor D1 by using [3H]-SCH-23,390 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 0.610nMAssay Description:Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMAssay Description:Inhibitory concentration against human Adenosine A3 receptor expressed in HEK293 cells using 0.1 nM [3H]AB-MECAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.760nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.840nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:Antidopamine activity in vitro by ability to displace [3H]spiperone from rat brain striatal preparations.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]- #NAME? from binding Dopamine receptor D1 in rat striatumMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of [3H]apomorphine binding to calf caudate membrane preparation (p4)More data for this Ligand-Target Pair
Affinity DataIC50: 1.01nMAssay Description:Tested for its affinity towards Dopamine receptor D1 in rat striatal membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Dopaminergic activity assessed in vitro for displacement of [3H]apomorphine from specific binding sites on rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:In vivo binding affinity of the compound against dopamine (D1) receptor in rat caudate-putamen tissue using [3H]-SCH-23,390 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Ability to displace [3H]-SCH- 23390 (0.2 nM) from corpus striatum of rat Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Ability to inhibit binding of [3H]-SCH- 23390 to Dopamine receptor D1 in rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In vitro binding affinity towards rat Dopamine receptor D1 by [3H]-SCH-23,390 displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibitory concentration for half-maximal displacement of 1.0 nM [3H]ADTN specific binding from rat striatal membranes using 10E-5 sulpirideMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Antidopamine activity in vitro by ability to displace [3H]spiperone from rat brain striatal preparations.More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of [3H]apomorphine binding to dopamine receptor of rat corpus striatumMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibitory concentration for half-maximal displacement of 1.0 nM [3H]ADTN specific binding from rat striatal membranes using 10E-5 sulpirideMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibitory concentration half-maximal displacement of 1.0 nM [3H]NPA specific binding from rat striatal membranes using 10e-5 (+/-)ADTNMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Antidopamine activity in vitro by ability to displace [3H]spiperone from rat brain striatal preparations.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Dopaminergic activity assessed in vitro for displacement of [3H]apomorphine from specific binding sites on rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:In vitro affinity for dopamine receptor by displacement of [3H]- apomorphine in rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Concentration inhibiting the specific binding of [3H]spiroperidol by 50%More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of [3H]spiperone binding to dopamine receptor from rat corpus striatal membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibitory concentration half-maximal displacement of 1.0 nM [3H]NPA specific binding from rat striatal membranes using 10e-5 (+/-)ADTNMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Binding affinity for dopamine receptor D1, activity is expressed as IC50 values.More data for this Ligand-Target Pair

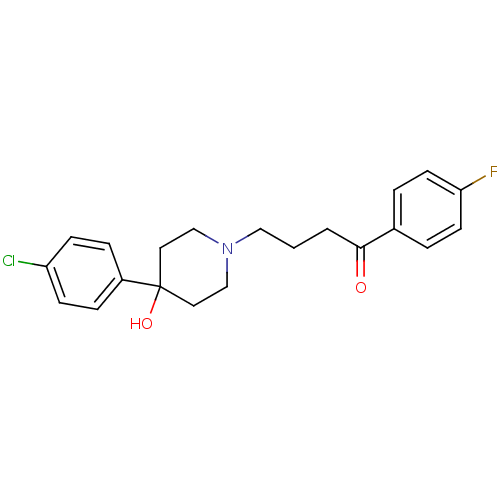
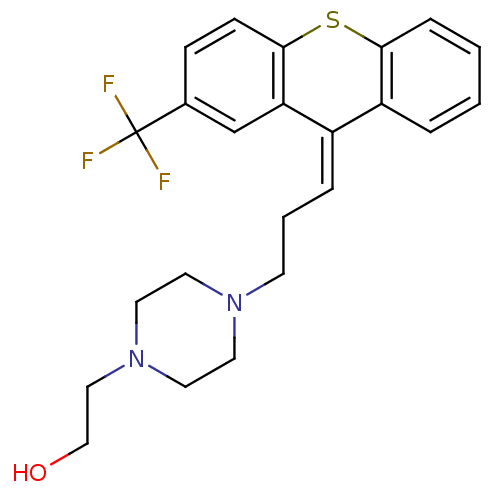
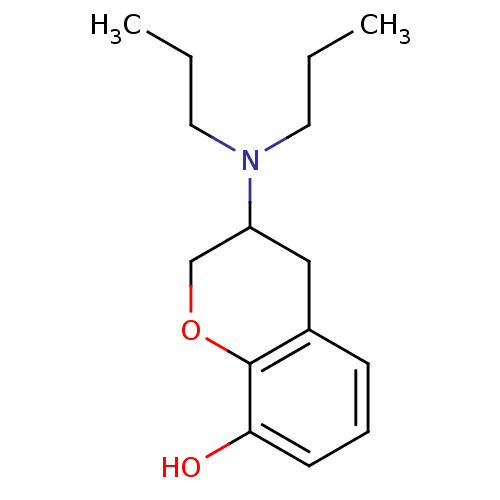
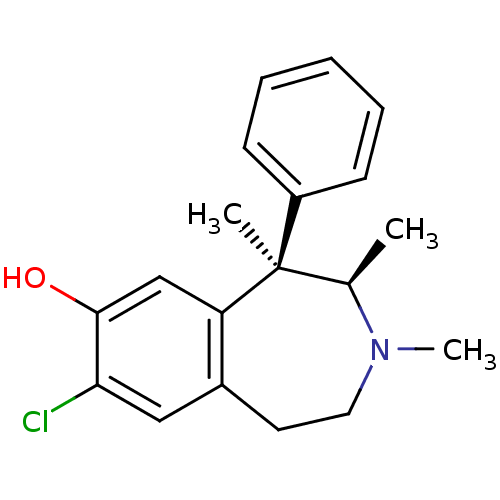
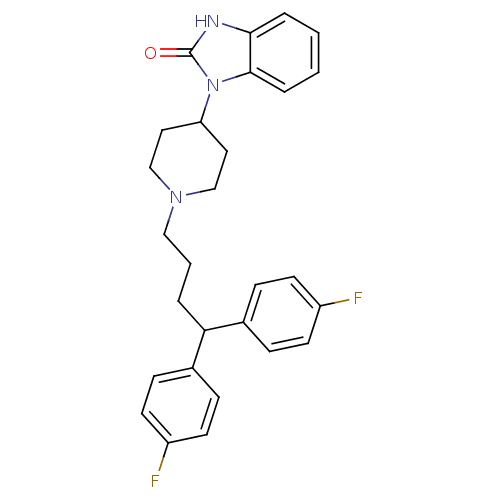
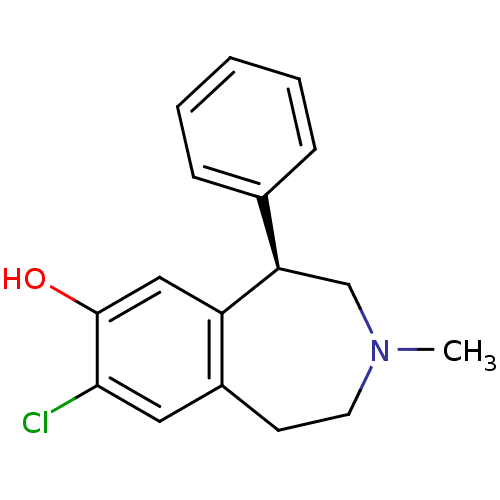
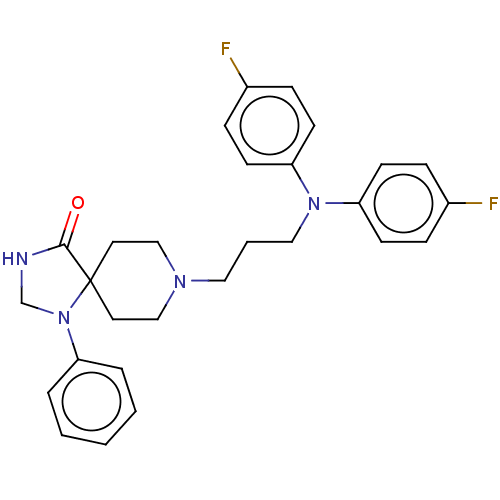
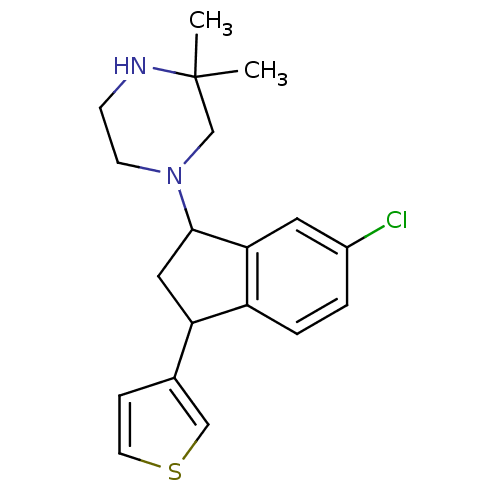
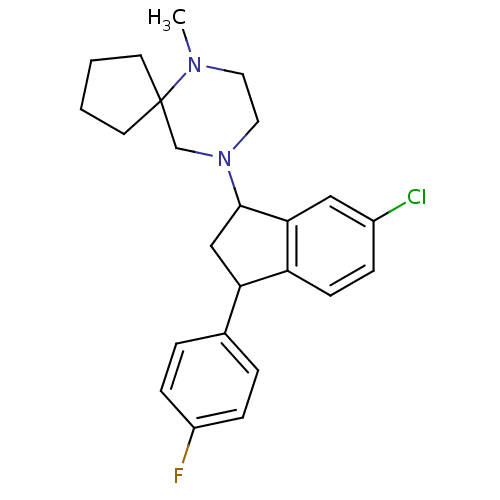
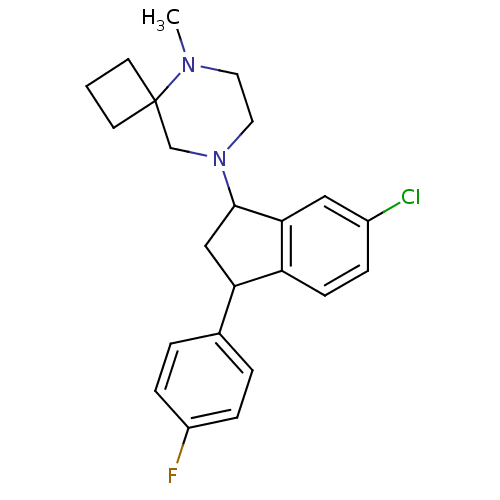
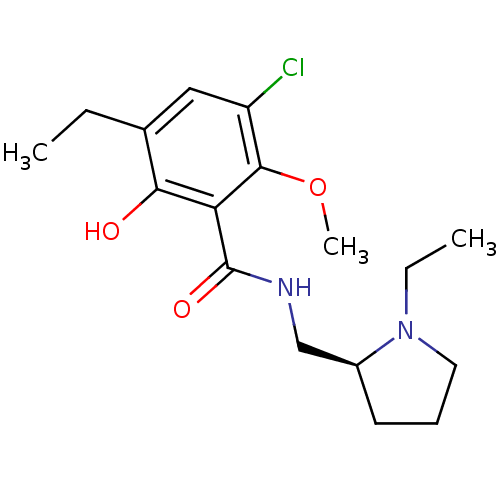
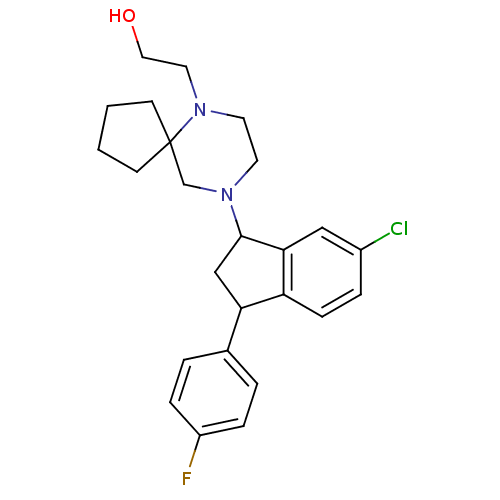
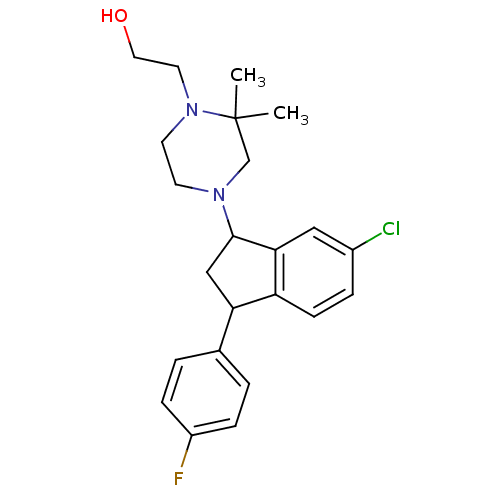
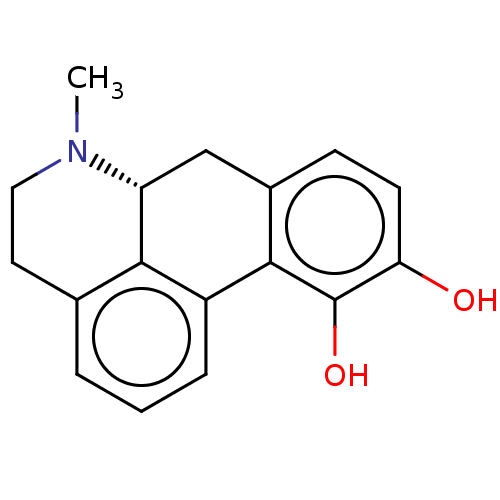
 3D Structure (crystal)
3D Structure (crystal)