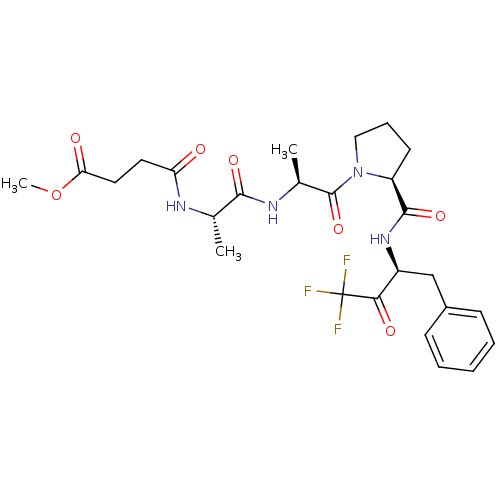
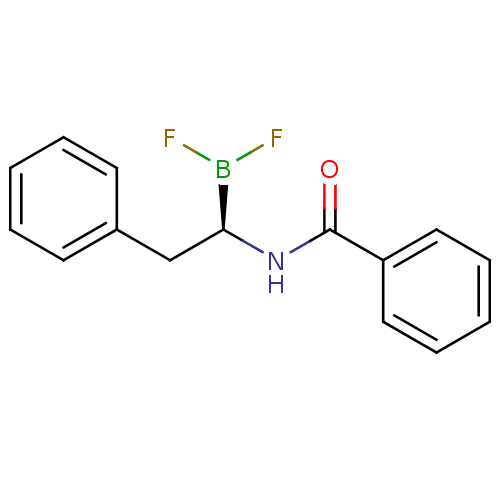
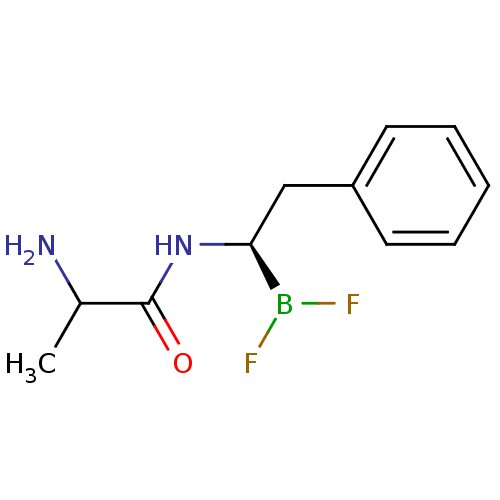
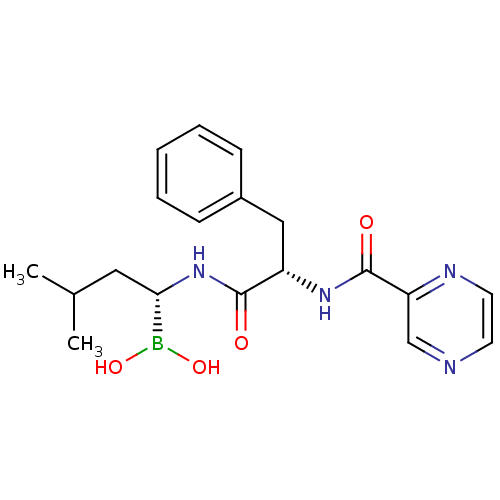
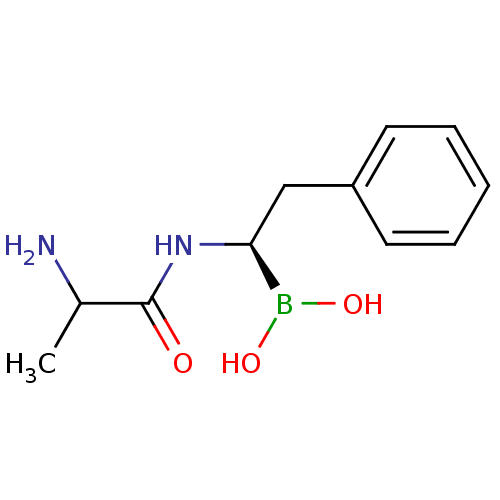
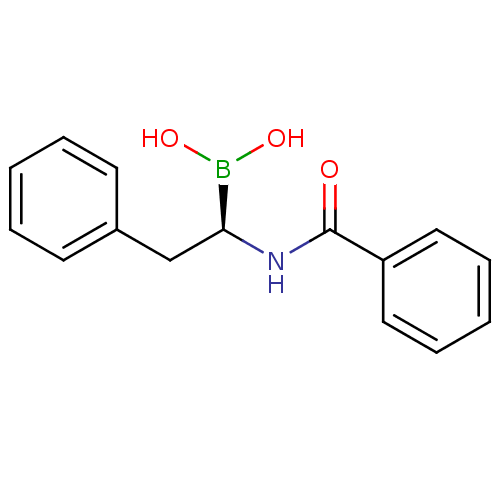
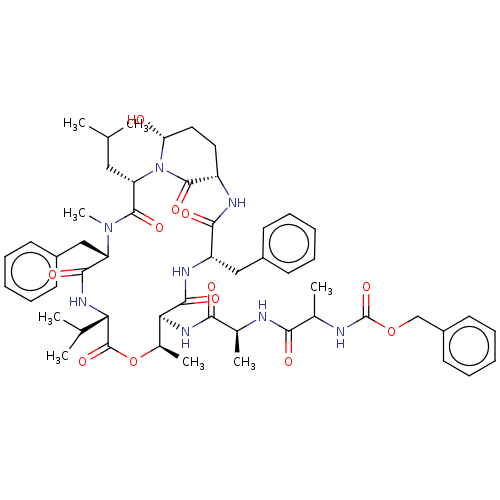
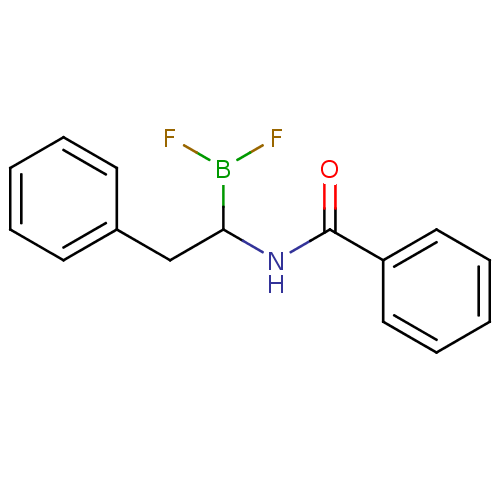
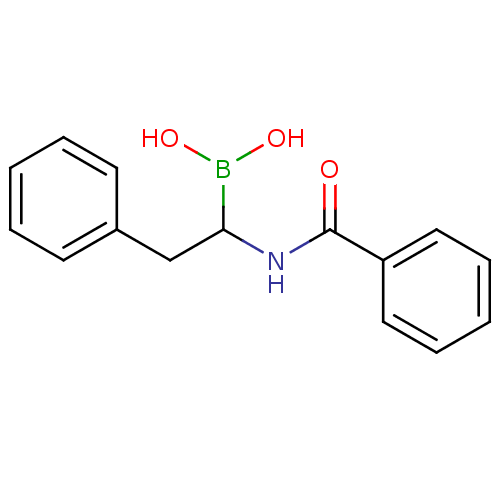
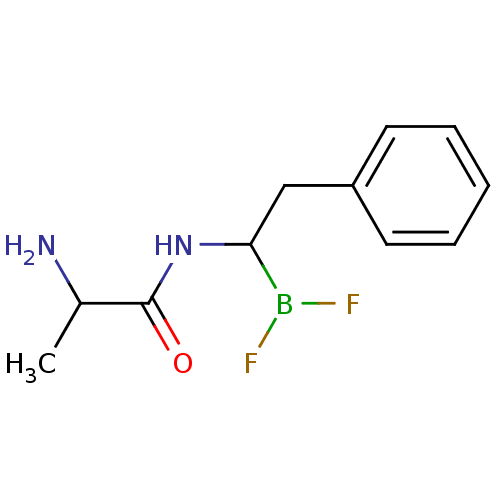
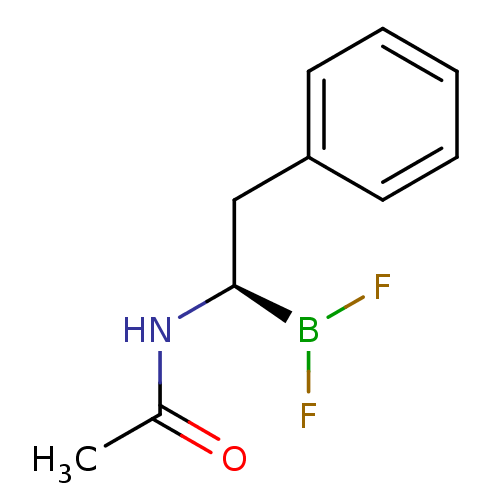
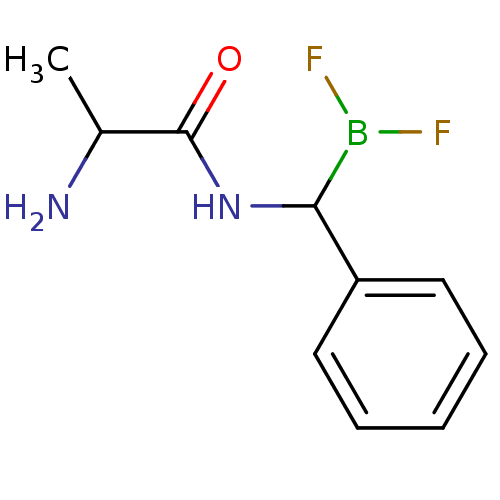
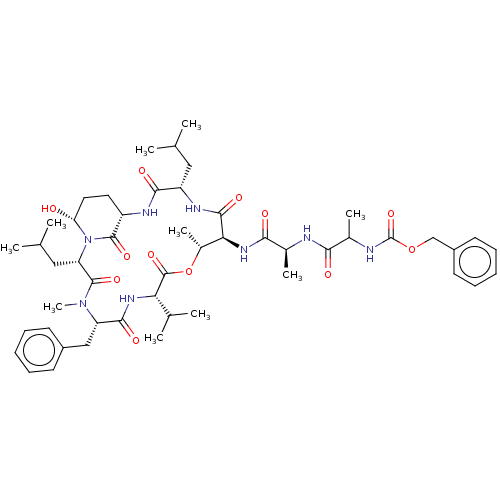
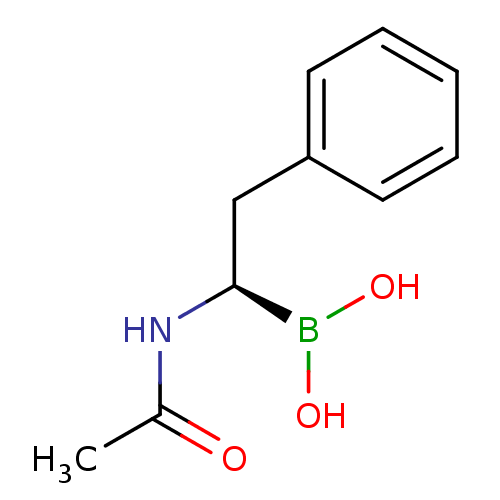
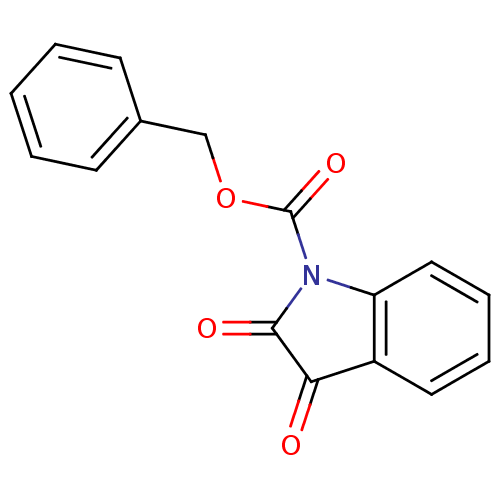
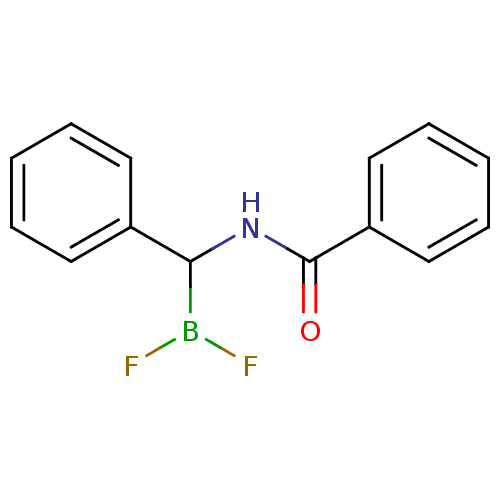
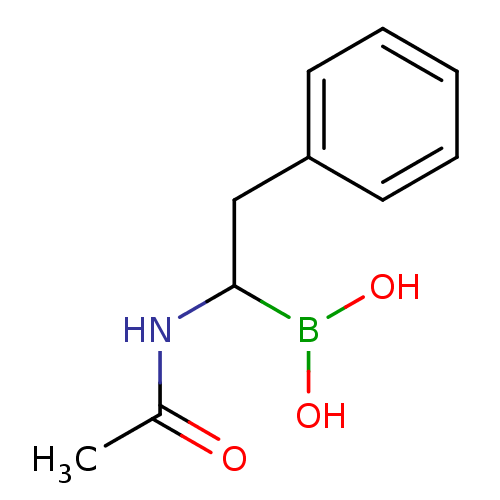
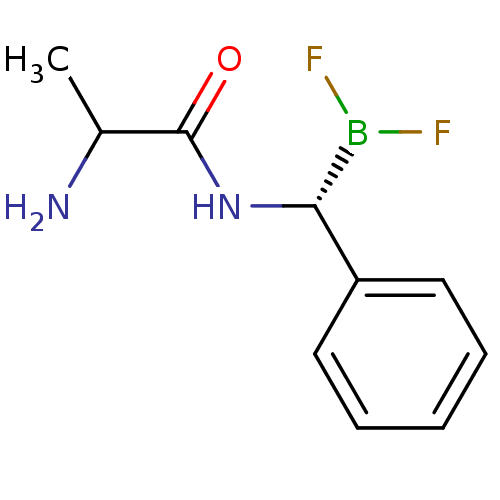
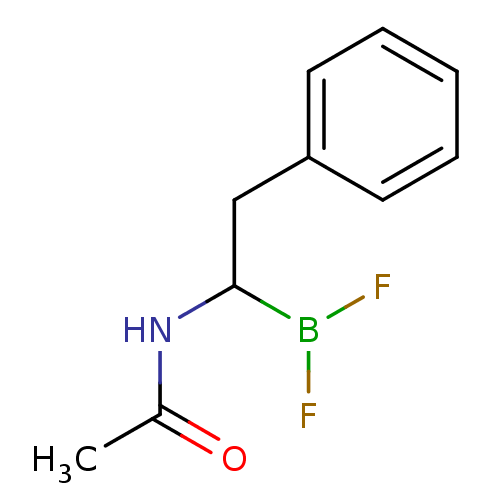
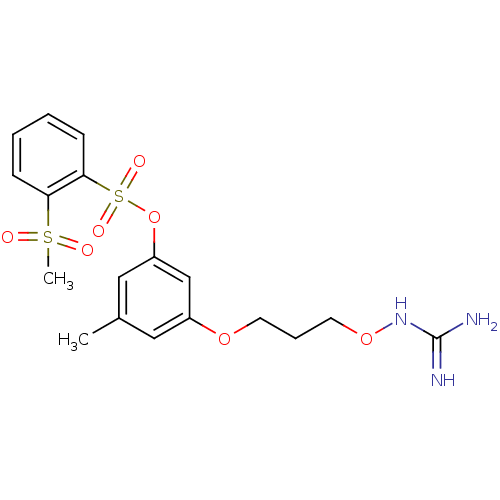
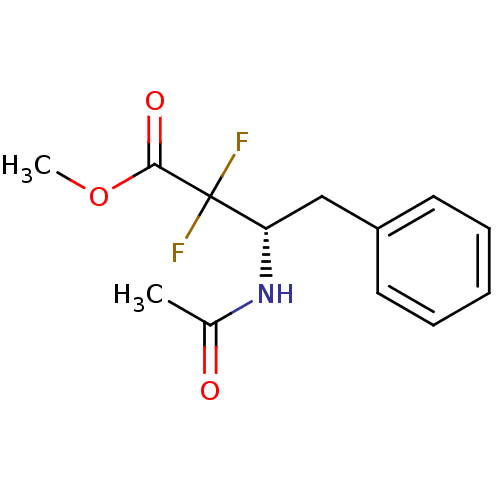
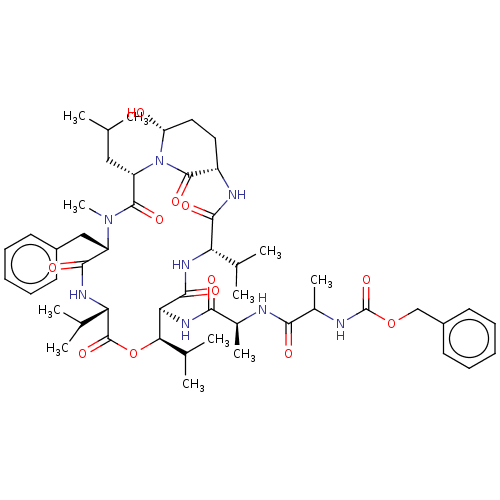
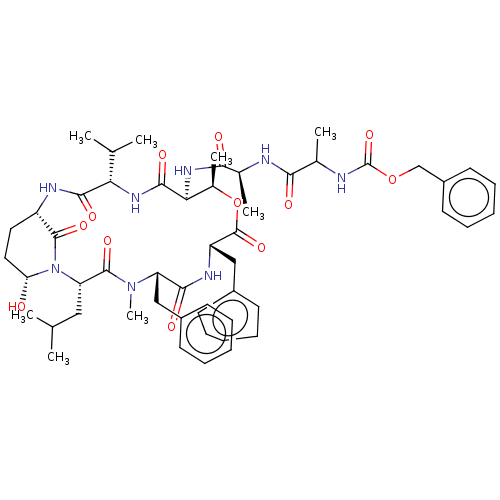
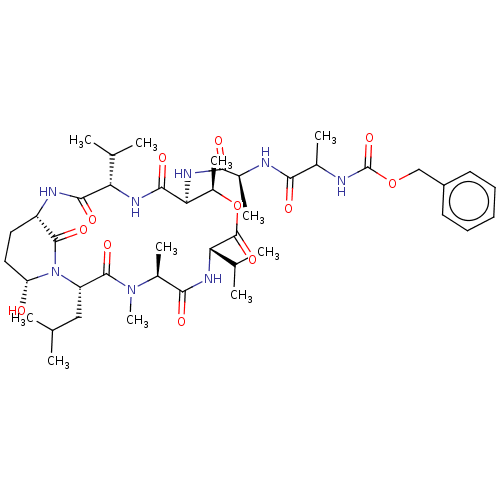
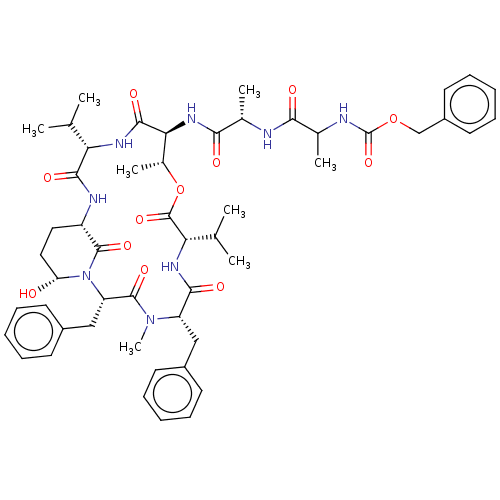
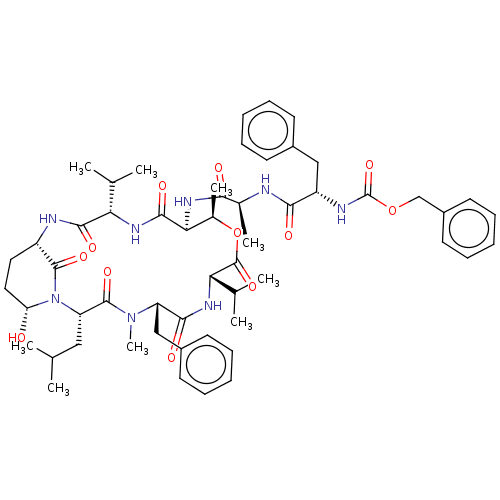
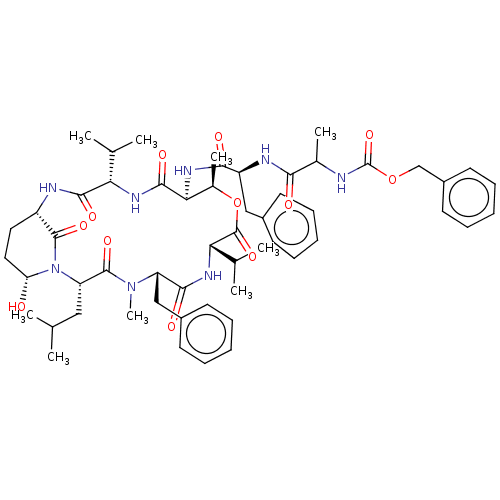
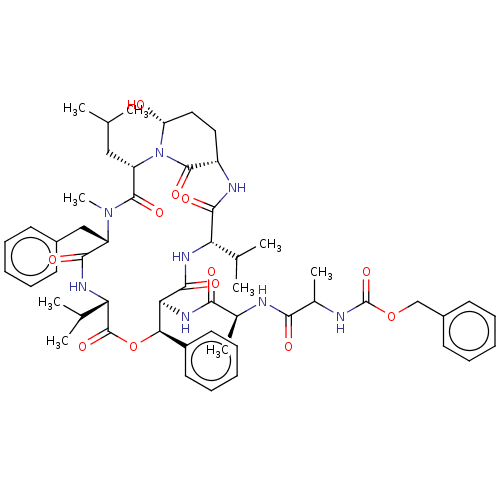
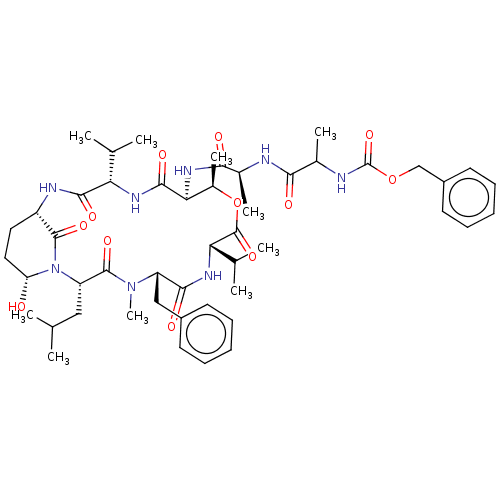
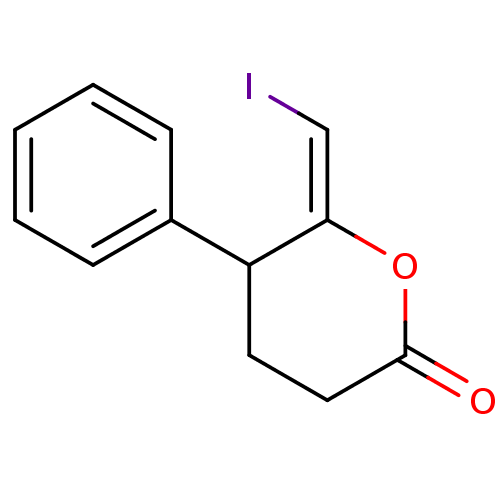
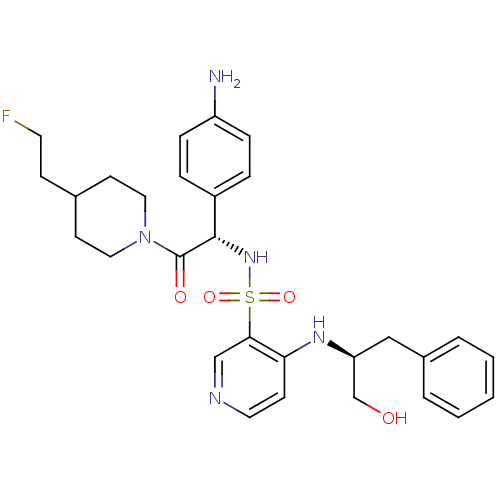
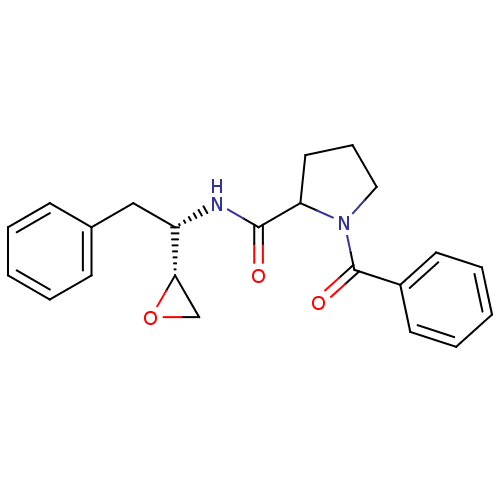
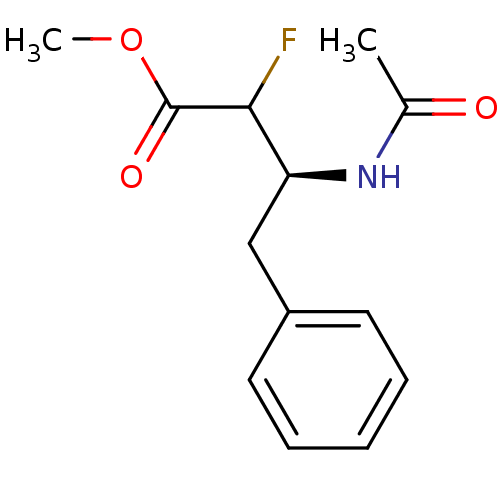
Affinity DataKi: 5.30nMAssay Description:Binding constant of the compound alpha-chymotrypsin was determined by competitive inhibition assay with BTEE as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Binding affinity against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Binding affinity against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 270nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Inhibitory activity against human ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 370nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: 650nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 650nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+3nMAssay Description:Inhibition constant against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 6.50E+3nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 6.50E+3nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 7.40E+3nMAssay Description:Competetive inhibition of alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: >2.20E+4nMAssay Description:In vitro inhibitory activity of the compound was tested against serine protease ChymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 3.40E+4nMpH: 7.8Assay Description:Reversible inhibitory activity of the compound toward alpha-chymotrypsin (5 nM concentration) at a pH of 7.8 by progress curve method.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut...More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nMAssay Description:Binding constant of the compound alpha-chymotrypsin was determined by competitive inhibition assay with Suc-Ala-Ala-Pro-Phe-pNA as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.15E+5nMAssay Description:Binding affinity of the compound was evaluated against against human chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 4.60E+5nMpH: 7.8Assay Description:Reversible inhibitory activity of the compound toward alpha-chymotrypsin (5 nM concentration) at a pH of 7.8 by progress curve method.More data for this Ligand-Target Pair
Affinity DataKi: 8.20E+5nMAssay Description:Compound was tested for inhibitory activity against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+6nMAssay Description:Inhibition constant of the compound against Alpha-chymotrypsin inhibitor was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 6.44E+6nMpH: 7.8Assay Description:Reversible inhibitory activity of the compound toward alpha-chymotrypsin (5 nM concentration) at a pH of 7.8 by progress curve method.More data for this Ligand-Target Pair
Affinity DataKi: 6.44E+6nMpH: 7.8Assay Description:Reversible inhibitory activity of the compound toward alpha-chymotrypsin (5 nM concentration) at a pH of 7.8 by progress curve method.More data for this Ligand-Target Pair