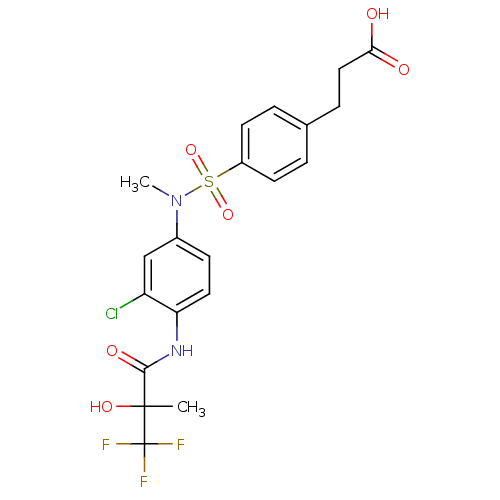
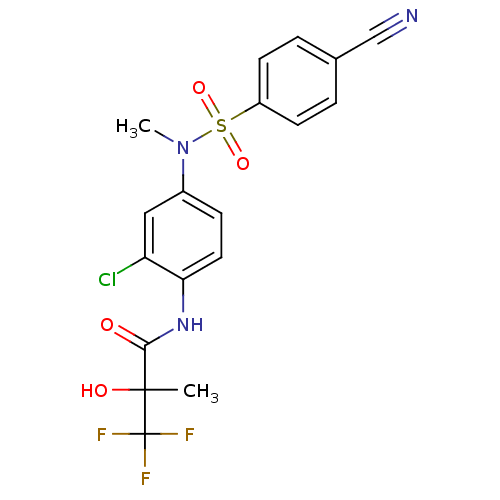
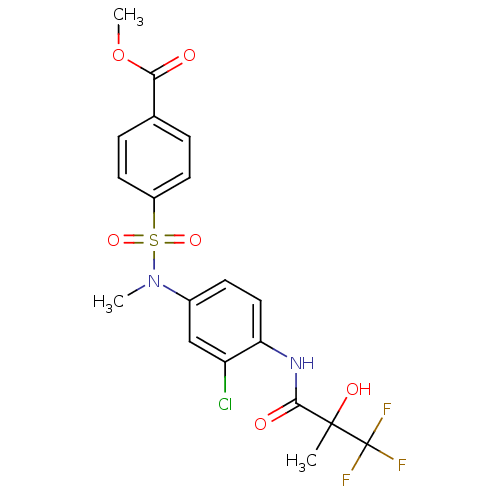
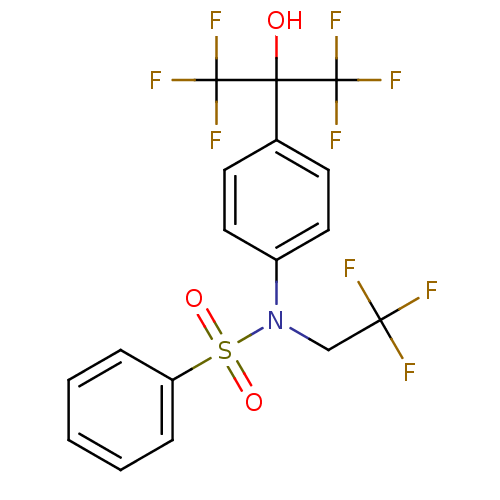
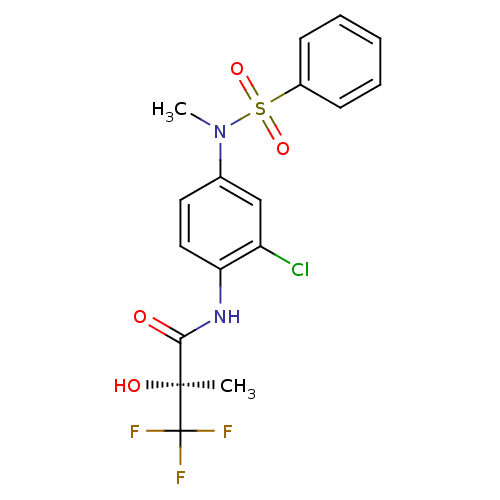
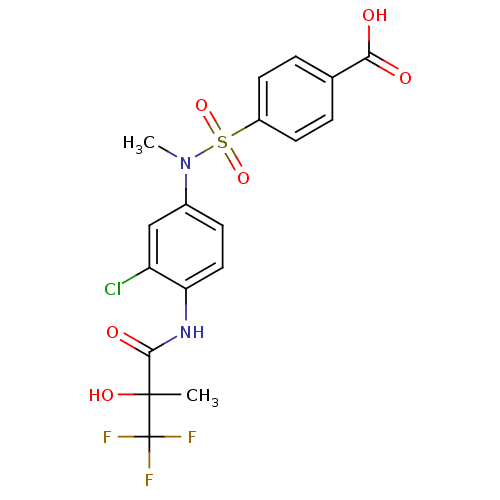
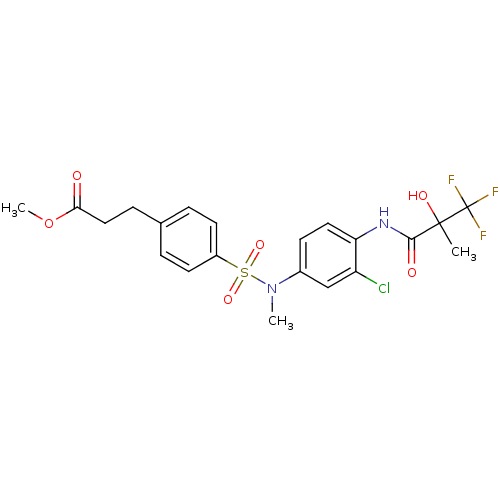
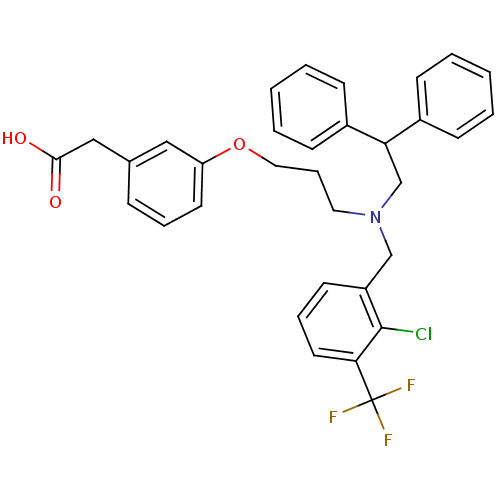
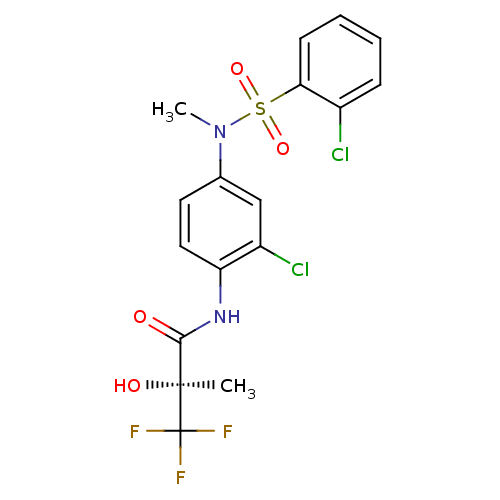
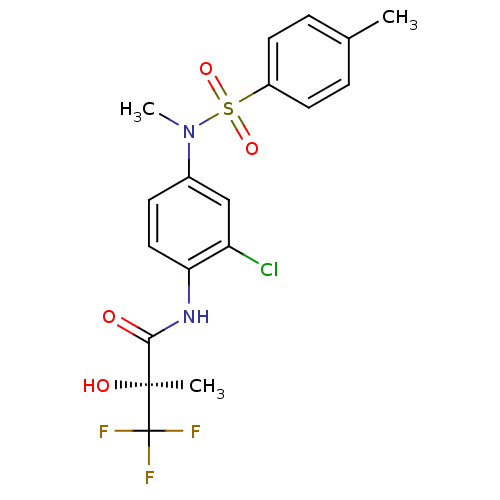
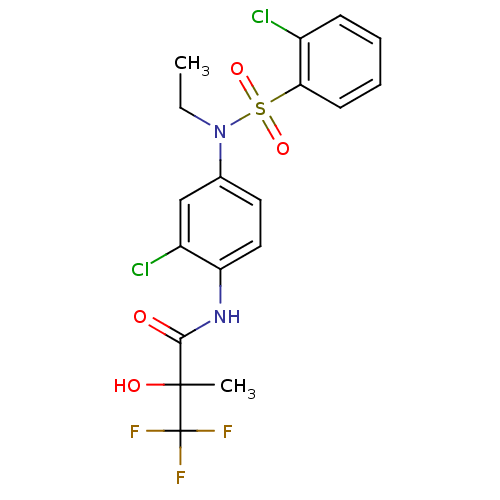
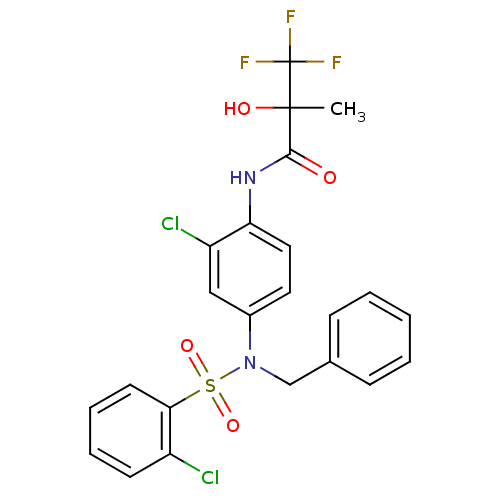
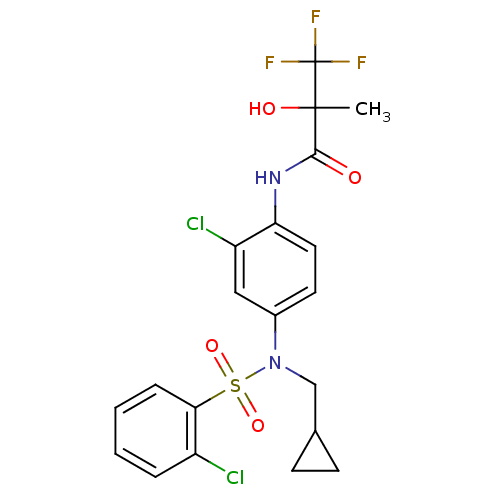
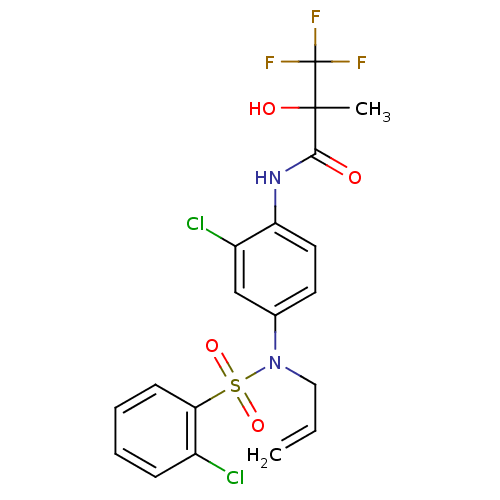
Affinity DataEC50: 286nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at human LXRalpha expressed in human SH-SY5Y cells cotransfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.70E+3nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.81E+3nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.60E+3nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.32E+3nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: 75nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 959nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.90E+3nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.50E+3nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 3.61E+3nMAssay Description:Agonist activity at human LXRalpha expressed in human SH-SY5Y cells cotransfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 5.00E+3nMAssay Description:Agonist activity at human LXRalpha expressed in human SH-SY5Y cells cotransfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 78nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: 813nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.59E+3nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at human LXRalpha expressed in human SH-SY5Y cells cotransfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at human LXRalpha expressed in human SH-SY5Y cells cotransfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: 626nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 1.00E+3nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.50E+3nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 1.97E+3nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: 7.50E+3nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 790nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 310nMAssay Description:Agonist activity at human LXRalpha expressed in human SH-SY5Y cells cotransfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 880nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: 400nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 470nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 5.00E+3nMAssay Description:Agonist activity at human LXRalpha expressed in human SH-SY5Y cells cotransfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 1.08E+3nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: 130nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 940nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at human LXRbeta expressed in human SH-SY5Y cells co-transfected with Gal4-LBD after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 580nMAssay Description:Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 280nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 280nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 3.30E+3nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 3.40E+3nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 3.10E+3nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Astrazeneca R&D SöDertäLje
Curated by ChEMBL
Affinity DataEC50: 3.50E+3nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair

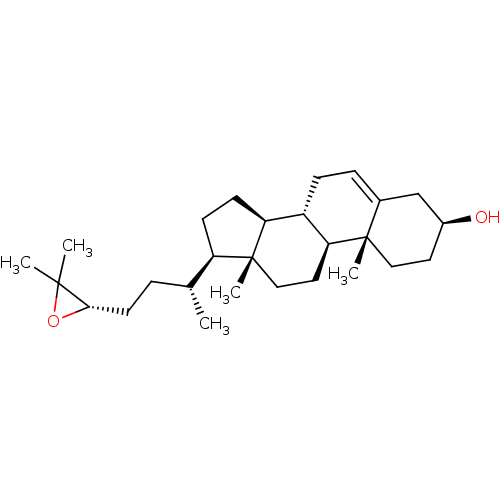
 3D Structure (crystal)
3D Structure (crystal)