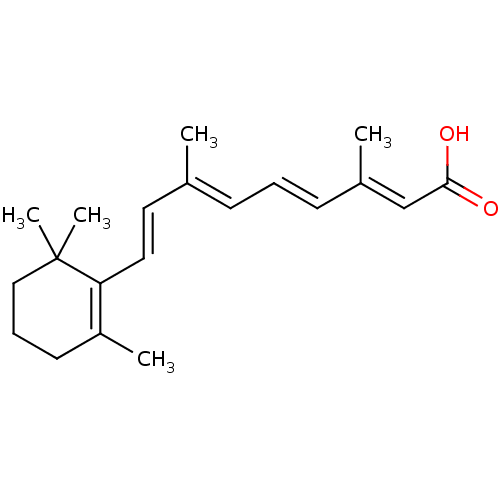
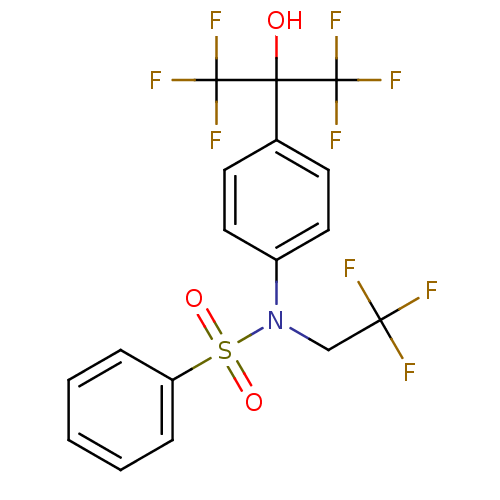
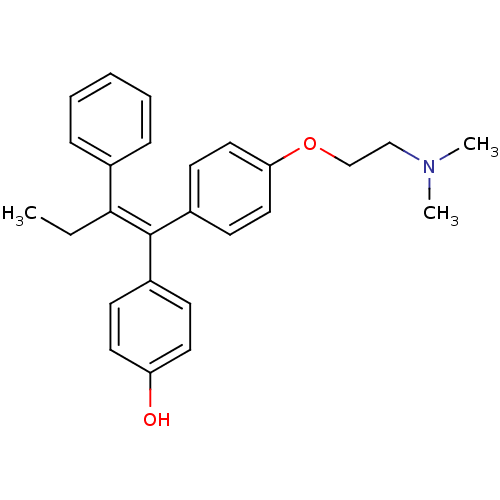
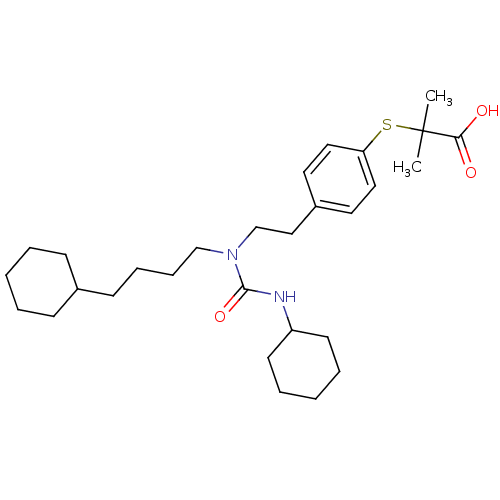
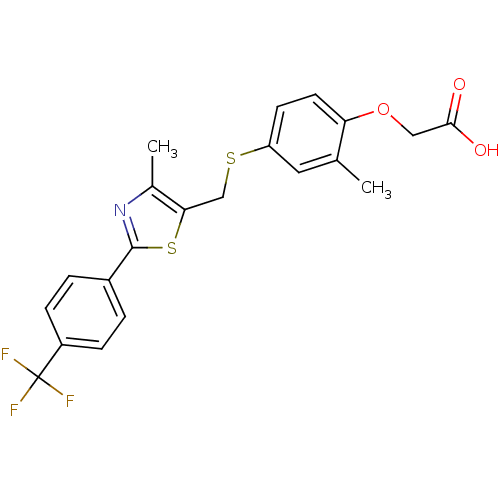
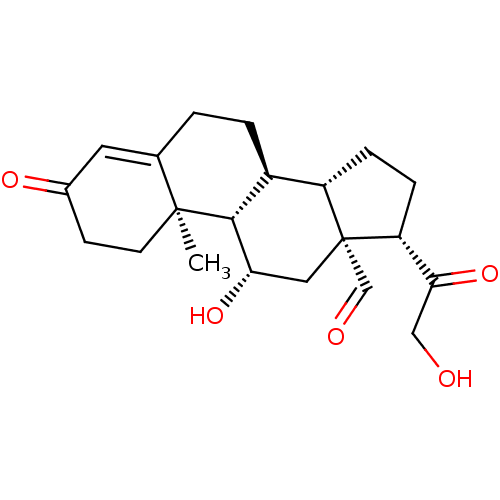
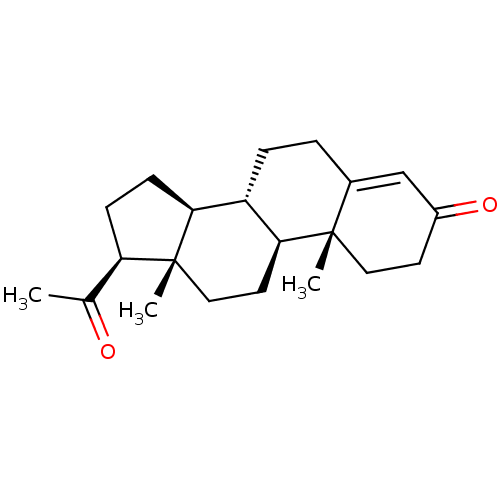
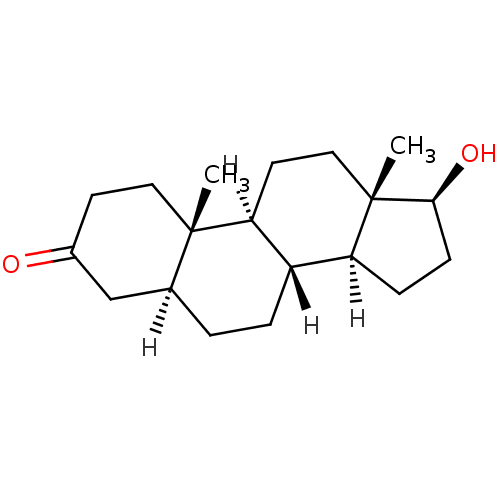
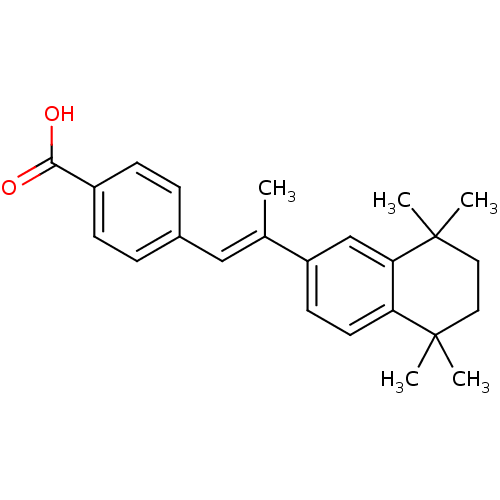
Affinity DataIC50: 7.90nMAssay Description:Inverse agonist activity at RORgammaT (unknown origin) by M1H assayMore data for this Ligand-Target Pair
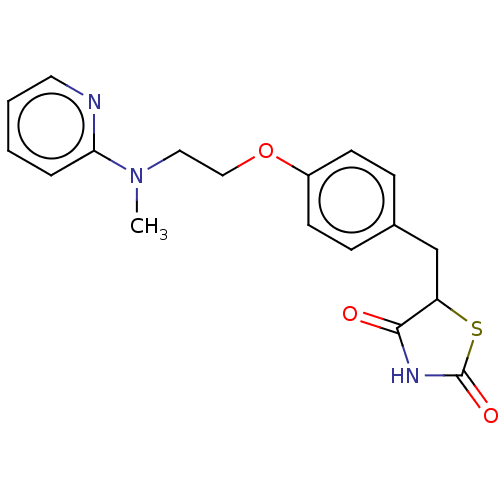
Affinity DataIC50: 10nMAssay Description:Inverse agonist activity at RORgammaT (unknown origin) by Jurkat cell based luciferase assayMore data for this Ligand-Target Pair
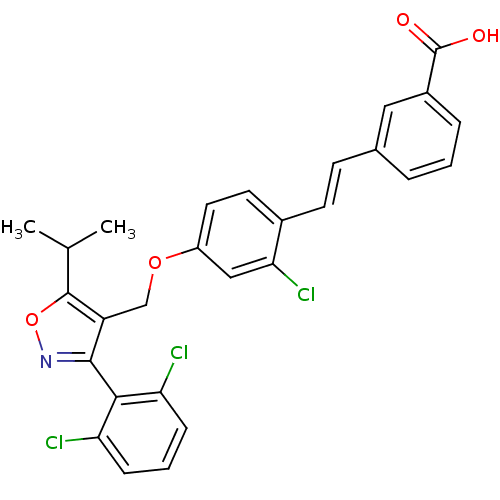
Affinity DataIC50: 13nMAssay Description:Inverse agonist activity at human recombinant N-terminally 6xHis-tagged RORgamma ligand binding domain (unknown origin) expressed in Escherichia coli...More data for this Ligand-Target Pair
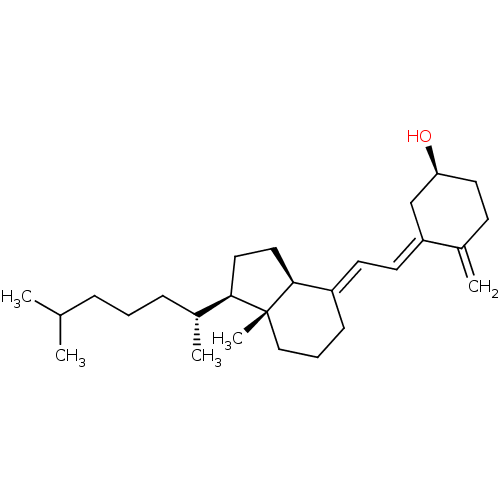
Affinity DataIC50: 16nMAssay Description:Inverse agonist activity at RORgammaT (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inverse agonist activity at human recombinant N-terminally 6xHis-tagged RORgamma ligand binding domain (unknown origin) expressed in Escherichia coli...More data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Inverse agonist activity at RORbeta (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inverse agonist activity at recombinant N-terminally GST-tagged RORbeta ligand binding domain (unknown origin) expressed in Escherichia coli incubate...More data for this Ligand-Target Pair
Affinity DataIC50: 79.4nMAssay Description:Inverse agonist activity at recombinant N-terminally GST-tagged RORbeta ligand binding domain (unknown origin) expressed in Escherichia coli incubate...More data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:Inverse agonist activity at RORbeta (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:Inverse agonist activity at RORgammaT (unknown origin) by Jurkat cell based luciferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inverse agonist activity at RORalpha (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inverse agonist activity at RORgammaT (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: 316nMAssay Description:Inverse agonist activity at human recombinant N-terminally 6xHis-tagged RORgamma ligand binding domain (unknown origin) expressed in Escherichia coli...More data for this Ligand-Target Pair
Affinity DataIC50: 501nMAssay Description:Inverse agonist activity at RORgammaT (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+3nMAssay Description:Agonist activity at human estrogen related receptor gamma expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inverse agonist activity at recombinant N-terminally GST-tagged RORbeta ligand binding domain (unknown origin) expressed in Escherichia coli incubate...More data for this Ligand-Target Pair
Affinity DataIC50: <5.01E+3nMAssay Description:Inverse agonist activity at RORbeta (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: <5.01E+3nMAssay Description:Inverse agonist activity at RORalpha (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: <5.01E+3nMAssay Description:Inverse agonist activity at RORalpha (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: <5.01E+3nMAssay Description:Inverse agonist activity at RORalpha (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: <5.01E+3nMAssay Description:Inverse agonist activity at RORbeta (unknown origin) by M1H assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inverse agonist activity at recombinant N-terminally GST-tagged RORbeta ligand binding domain (unknown origin) expressed in Escherichia coli incubate...More data for this Ligand-Target Pair
Affinity DataIC50: 2.51E+4nMAssay Description:Inverse agonist activity at recombinant N-terminally GST-tagged RORbeta ligand binding domain (unknown origin) expressed in Escherichia coli incubate...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Phenex Pharmaceuticals
Curated by ChEMBL
Phenex Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 0.890nMAssay Description:Agonist activity at human PPARalpha expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Phenex Pharmaceuticals
Curated by ChEMBL
Phenex Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human PPARbeta expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Phenex Pharmaceuticals
Curated by ChEMBL
Phenex Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Agonist activity at human PPARgamma expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 85nMAssay Description:Agonist activity at human LXRbeta expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 26nMAssay Description:Agonist activity at human FXR expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.210nMAssay Description:Agonist activity at human VDR expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 3(Homo sapiens (Human))
Phenex Pharmaceuticals
Curated by ChEMBL
Phenex Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 75nMAssay Description:Agonist activity at human CAR expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 10nMAssay Description:Agonist activity at human RXRalpha expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0240nMAssay Description:Agonist activity at human ERalpha expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0850nMAssay Description:Agonist activity at human ERbeta expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.30nMAssay Description:Agonist activity at human GCR expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human MCR expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Agonist activity at human PRGR expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human androgen receptor expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Phenex Pharmaceuticals
Curated by ChEMBL
Phenex Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 260nMAssay Description:Agonist activity at human pregnane X receptor expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human RARalpha expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Phenex Pharmaceuticals
Curated by ChEMBL
Phenex Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 80nMAssay Description:Agonist activity at human pregnane X receptor expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.410nMAssay Description:Agonist activity at human thyroid hormone receptor alpha expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)