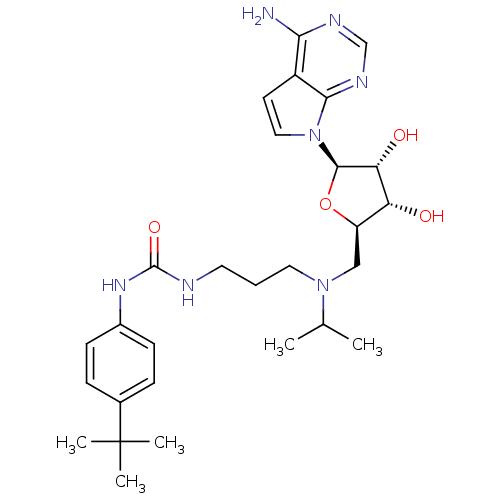
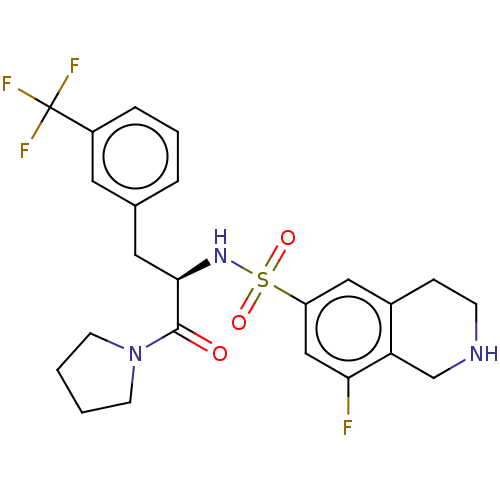
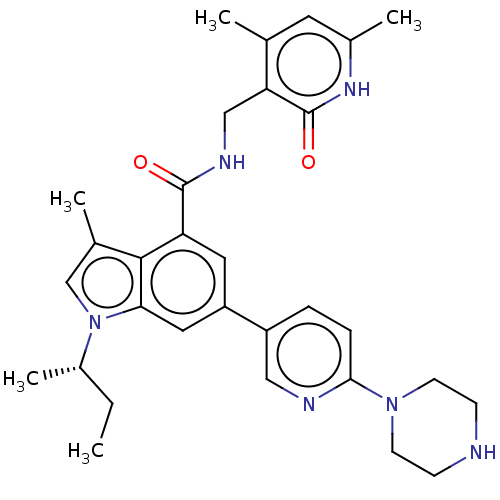
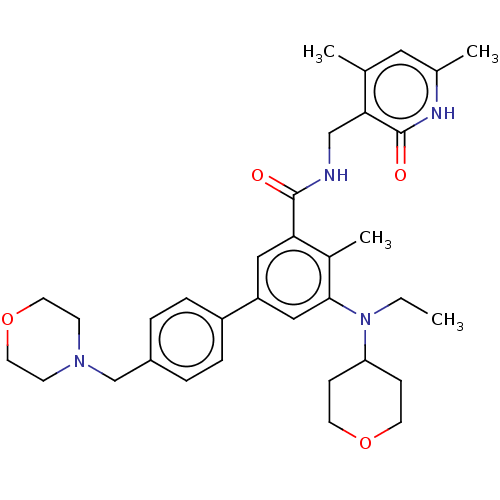
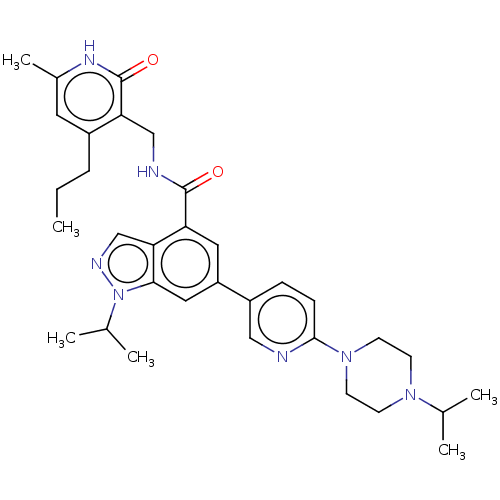
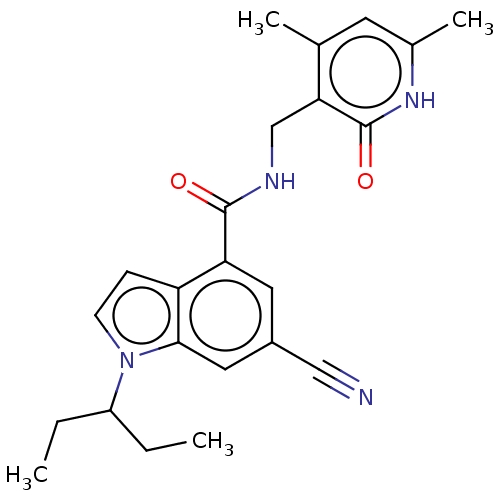
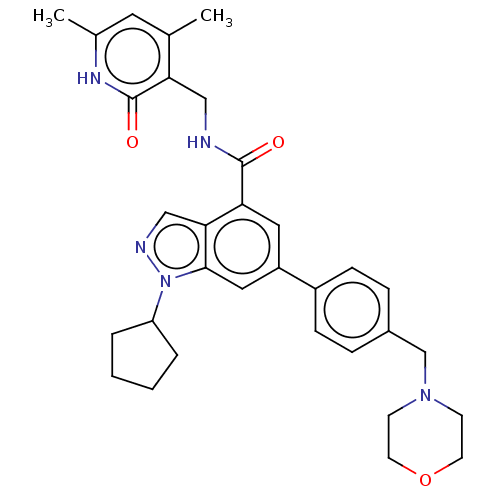
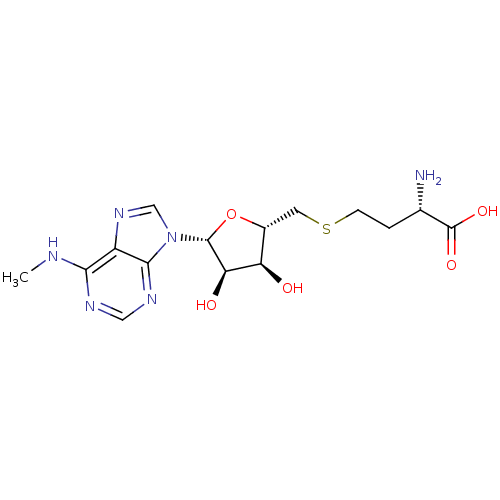
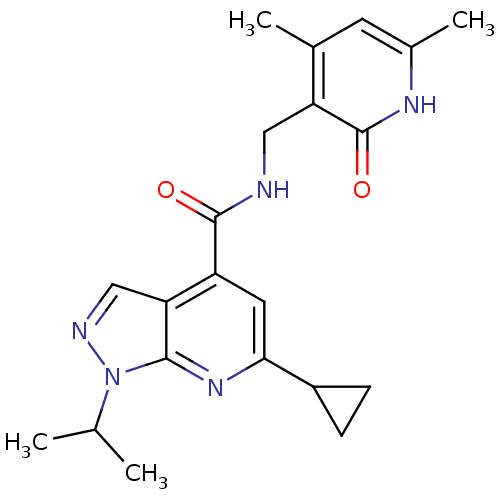
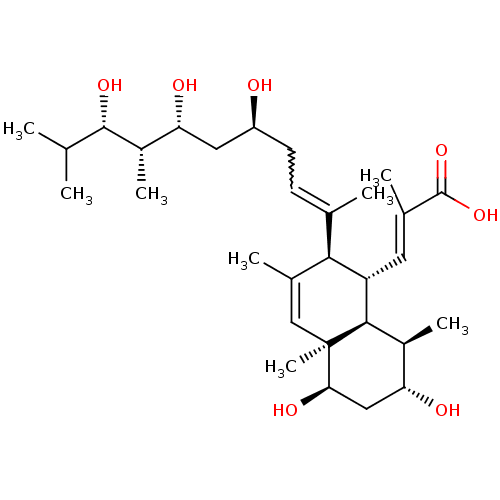
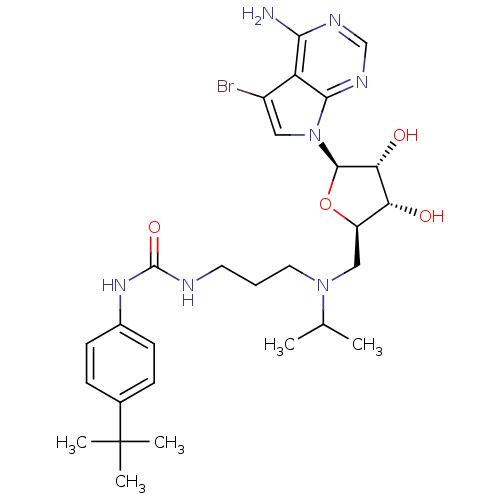
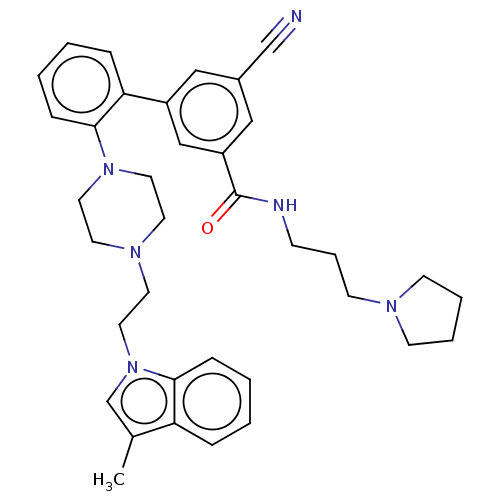
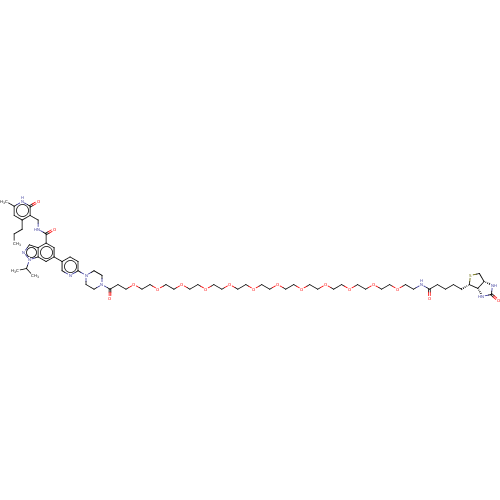
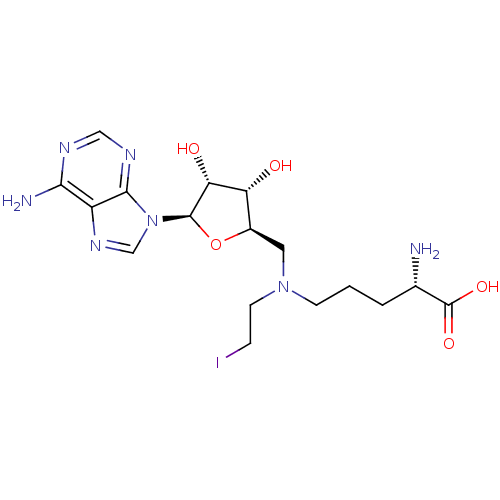
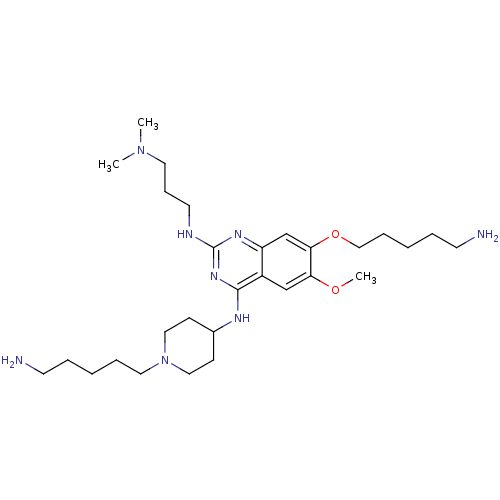
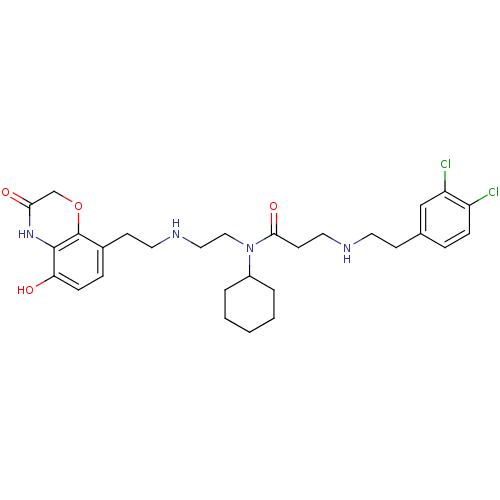
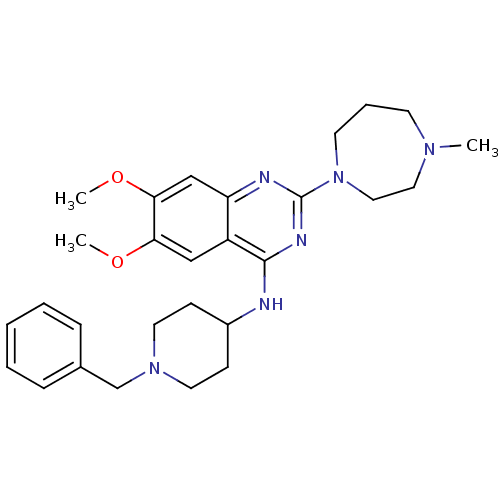
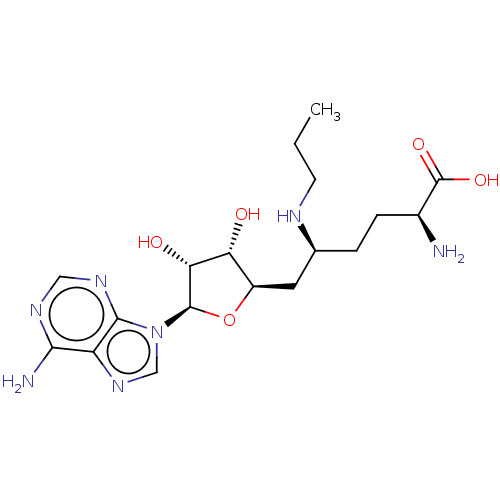
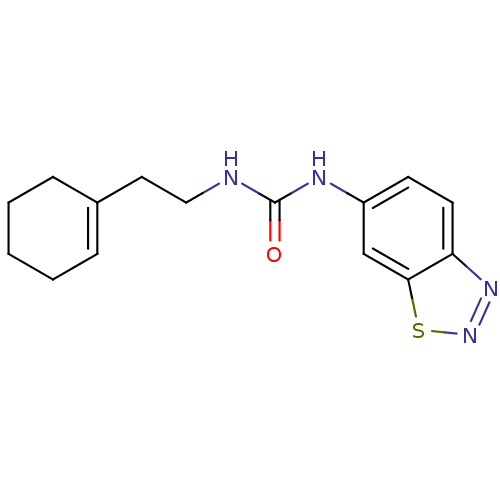
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.0800nMAssay Description:Inhibition of human DOT1L using oligo-nucleosome/[3H]-SAM as substrate preincubated for 30 mins followed by substrate addition measured after 120 min...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Binding affinity to human DOT1L after 120 minsMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibition of human DOT1L using oligo-nucleosome/[3H]-SAM as substrate preincubated for 30 mins followed by substrate addition measured after 120 min...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase SETD7(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.330nMAssay Description:Inhibition of full-length human SETD7 expressed in Escherichia coli BL21 (DE3) using biotinylated histone H3 (1 to 25) as substrate after 1 hr by Fla...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human EZH2 A677G mutant assessed as H3K27me0 level after 30 mins by scintillation counting analysis in presence of [3H]-SAMMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human wild-type EZH2 assessed as H3K27me0 level after 30 mins by scintillation counting analysis in presence of [3H]-SAMMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human EZH2 Y641C mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAMMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human EZH2 A677G mutant assessed as H3K27me1 level after 30 mins by scintillation counting analysis in presence of [3H]-SAMMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human EZH2 Y641F mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAMMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human EZH2 Y641H mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAMMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human EZH2 Y641N mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAMMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human EZH2 Y641S mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAMMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Inhibition of wild-type human EZH2 by flash plate assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 4.60nMAssay Description:Competitive inhibition of EZH2 (unknown origin) using biotinylated-histone H3 (1 to 24) as substrate by Lineweaver-Burk plot analysis in presence of ...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Inhibition of N-terminal FLAG tagged full-length human EZH2 expressed in baculovirus infected Sf9 cells using H3K27 as substrate in presence of SAMMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Competitive inhibition of human EZH2 using SAM and histone H3 (16 to 30) as substrate preincubated for 30 mins followed by substrate addition measure...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 290nMAssay Description:Competitive inhibition of human DOT1L (1 to 472) using oligo-nucleosome as substrate preincubated for 10 mins followed by substrate addition measured...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Non-competitive inhibition of human EZH2 using [3H]-SAM as substrate in presence of H3K27AMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Competitive inhibition of human EZH2 using H3K27A as substrate in presence of SAMMore data for this Ligand-Target Pair
TargetN-lysine methyltransferase KMT5A(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Competitive inhibition of SETD8 (unknown origin) using H4 (1 to 24) as substrate assessed as incorporation of [3H]-methyl group from [3H-Me]-SAM to p...More data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: >2.00E+4nMAssay Description:Competitive inhibition of human CARM1 using oligo-nucleosome as substrate preincubated for 10 mins followed by substrate addition measured after 30 m...More data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 1 [11-371](Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: >2.00E+4nMAssay Description:Competitive inhibition of PRMT1 (unknown origin) using histone-H4 as substrate preincubated for 10 mins followed by substrate addition measured after...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase SUV39H1(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: >2.00E+4nMAssay Description:Competitive inhibition of SUV39H1 (unknown origin) using histone-H3 (1 to 21) as substrate preincubated for 10 mins followed by substrate addition me...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataKi: >2.00E+4nMAssay Description:Competitive inhibition of G9a (unknown origin) using histone-H3 (1 to 21) as substrate preincubated for 10 mins followed by substrate addition measur...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human DOT1L (1 to 416) using [3H]-SAM, SAM and nucleosome as substrate assessed as incorporation of radioactivity into nucl...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase SETD7(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of full-length human SETD7 expressed in Escherichia coli BL21 (DE3) using biotinylated histone H3 (1 to 25) as substrate after 1 hr by Fla...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of DOT1L in human MV4-11 cells expressing MLL-AF4 assessed as reduction of H3K79me2 level after 4 days by ELISA methodMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Inhibition of DOT1L in human MV4-11 cells expressing MLL-AF4 assessed as cell growth inhibition after 14 days by Guava Viacount assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 8.80nMAssay Description:Inhibition of DOT1L in human MCF10A cells assessed as reduction of H3K79 levelMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: <10nMAssay Description:Inhibition of EZH2 (unknown origin) using biotinylated-histone H3 (1 to 24) as substrate after 1 hr by scintillation proximity assay in presence of [...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of N-terminal FLAG tagged human EZH2 Y641F mutant expressed in baculovirus infected Sf9 cells using H3K27 as substrate after 120 mins by m...More data for this Ligand-Target Pair
TargetN-lysine methyltransferase SMYD2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: <15nMAssay Description:Inhibition of SMYD2 (unknown origin)More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of N-terminal FLAG tagged full-length human EZH2 expressed in baculovirus infected Sf9 cells using H3K27 as substrate after 120 mins by ma...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of EZH2 (unknown origin) using biotinylated-histone H3 (1 to 24) as substrate after 1 hr by scintillation proximity assay in presence of [...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of EZH2 (unknown origin) using biotinylated-histone H3 (1 to 24) as substrate after 1 hr by scintillation proximity assay in presence of [...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of human DOT1L (1 to 472) using [3H]-SAM/oligo-nucleosome as substrate preincubated for 10 mins followed by substrate addition measured af...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of wild-type EZH2 in human OCI-LY19 cells assessed as reduction of H3K27me3 level after 96 hrs by ELISA assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 84nMAssay Description:Inhibition of DOT1L in human MCF10A cells assessed as reduction of H3K79 levelMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT1(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of recombinant human GLP (951 to 1235) using Histone H3 peptide (1 to 15) as substrate preincubated on ice for 10 mins followed by 5 mins ...More data for this Ligand-Target Pair
TargetN-lysine methyltransferase SMYD2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of recombinant human full-length SMYD2 (1 to 433) using biotin-aminohexanoyl GSRAHSSHLKSKKGQSTSRH as substrate after 75 mins by AlphaScree...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 124nMAssay Description:Inhibition of EZH2 in human MCF10A cells assessed as reduction of H3K27me3 level after 72 hrs by Western blot analysisMore data for this Ligand-Target Pair
TargetN-lysine methyltransferase KMT5A(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of N-terminal His6-tagged human SETD8 (191 to 352) assessed as incorporation of [3H]-methyl group from [3H-Me]-SAM to biotinylated H4K20 p...More data for this Ligand-Target Pair
TargetN-lysine methyltransferase KMT5A(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibition of N-terminal His6-tagged human SETD8 (191 to 352) assessed as incorporation of [3H]-methyl group from [3H-Me]-SAM to biotinylated H4K20 p...More data for this Ligand-Target Pair
TargetN-lysine methyltransferase SMYD2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of SMYD2 in human KYSE-150 cells assessed as reduction of monomethylation of p53 K370 by sandwich ELISA methodMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 620nMAssay Description:Inhibition of human EZH2 using SAM and histone H3 (16 to 30) as substrate preincubated for 30 mins followed by substrate addition measured after 90 m...More data for this Ligand-Target Pair
TargetN-lysine methyltransferase KMT5A(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 700nMAssay Description:Inhibition of N-terminal His6-tagged human SETD8 (191 to 352) assessed as incorporation of [3H]-methyl group from [3H-Me]-SAM to biotinylated H4K20 p...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT1(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 700nMAssay Description:Inhibition of N-terminal hexahistidine-tagged SET domain of human GLP (951 to 1235) expressed in Escherichia coli BL21 (DE3) using Histone H3 peptide...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase SETD2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of human SETD2 (1347 to 1711) using histamine H3 (20 to 50) as substrate assessed as transfer of [3H]-Me of [3H-Me]-SAM to peptide substra...More data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 3 [N508S](Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of recombinant human PRMT3 (211 to 531) using histone-4 (1 to 24) as substrate by S-adenosylhomocysteine hydrolase-coupled assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School Of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of G9a (685 to 1000) (unknown origin) assessed as H3K9me2 level by mass spectrophotometric analysisMore data for this Ligand-Target Pair

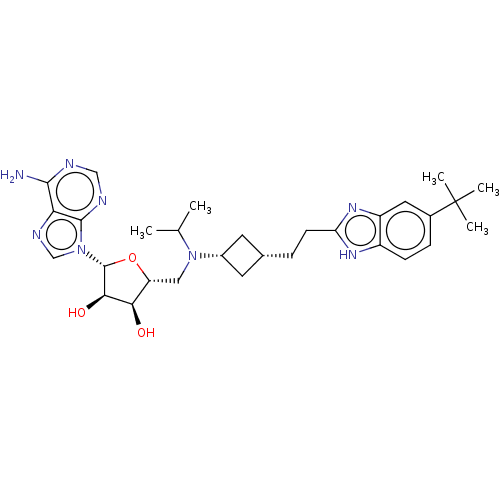
 3D Structure (crystal)
3D Structure (crystal)