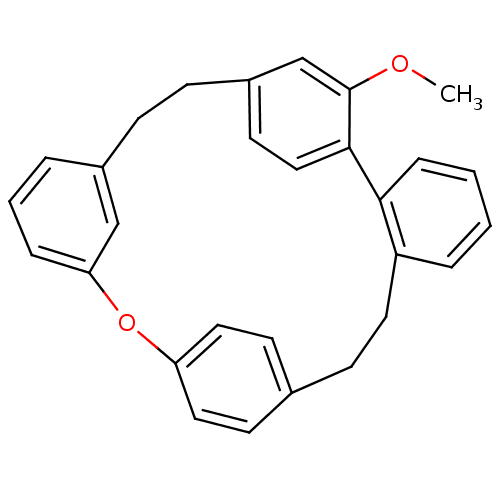
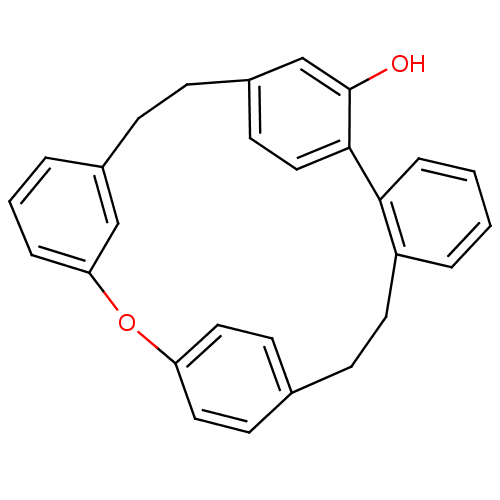
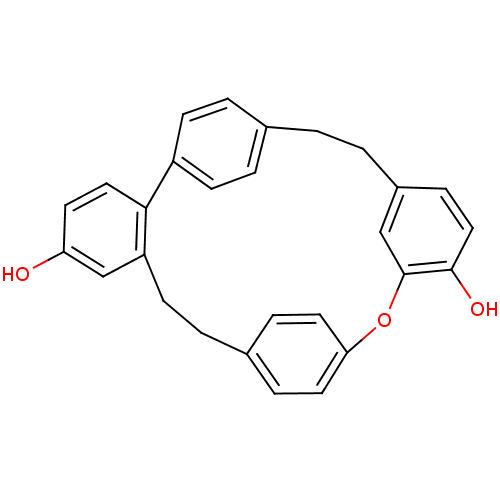
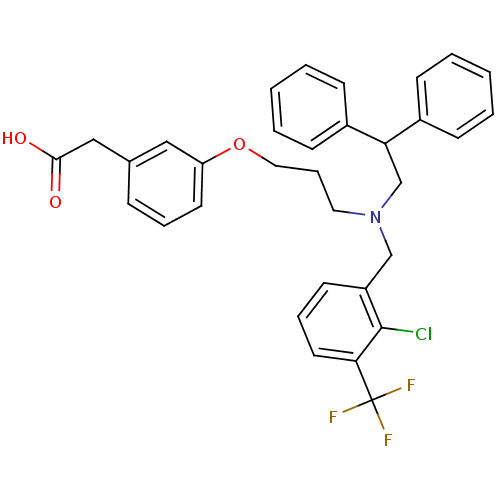
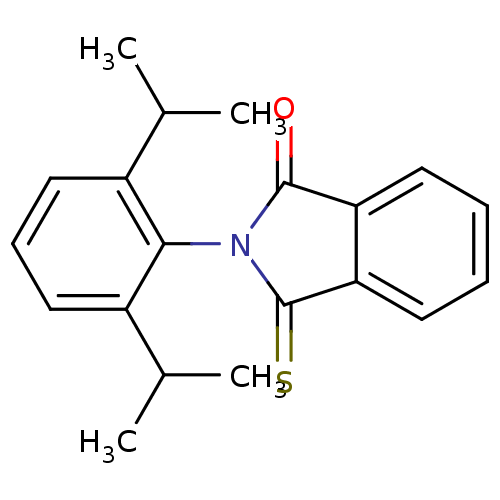
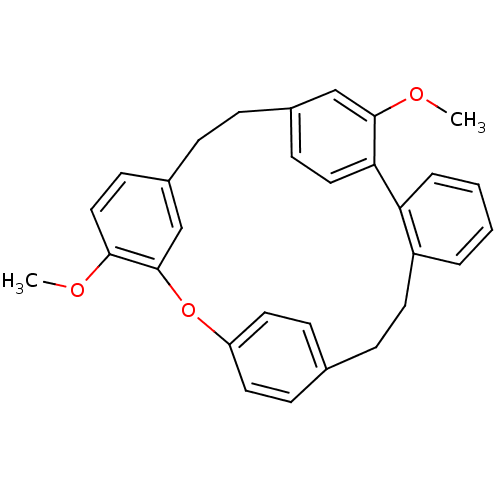
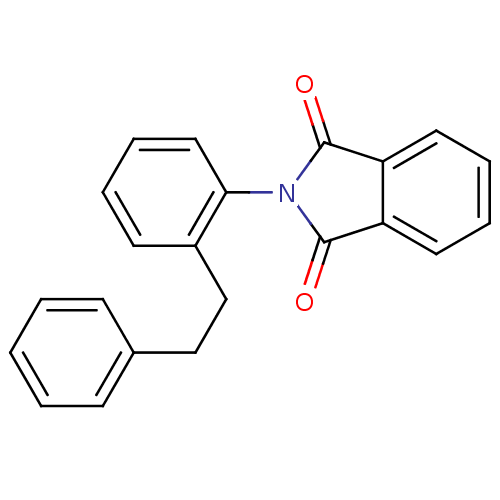
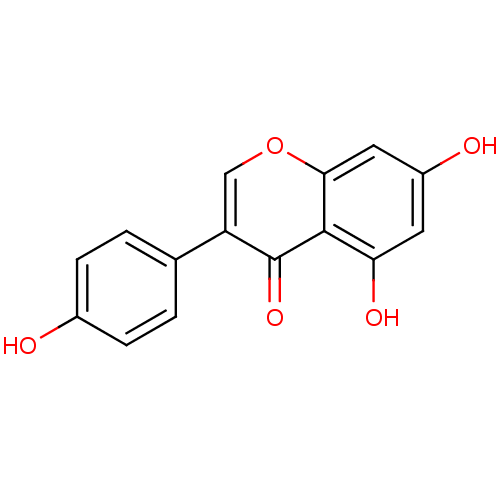
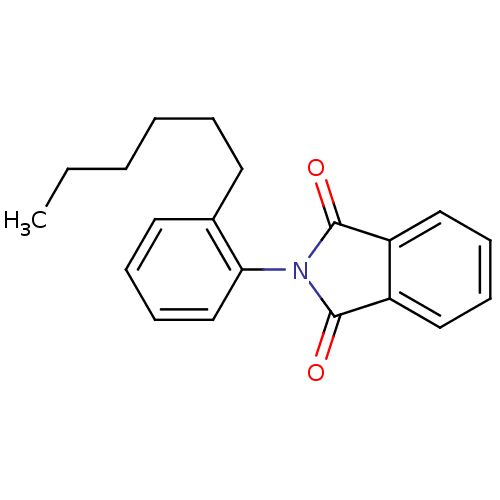
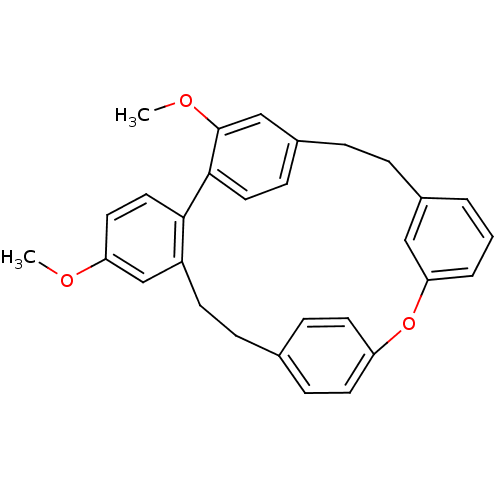
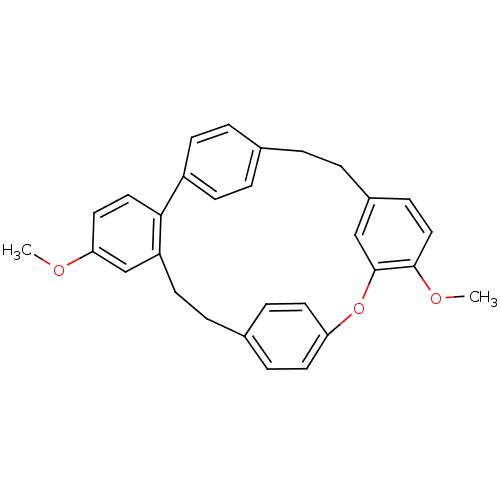
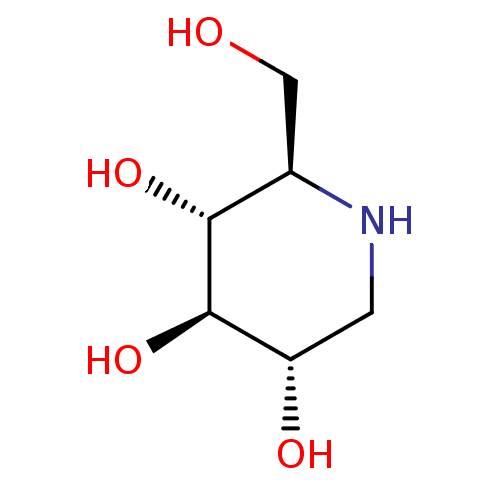
Affinity DataIC50: 2.50E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+3nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 8.40E+3nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 8.60E+3nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 9.90E+3nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.62E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 2.47E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 6.70E+4nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 7.70E+4nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair