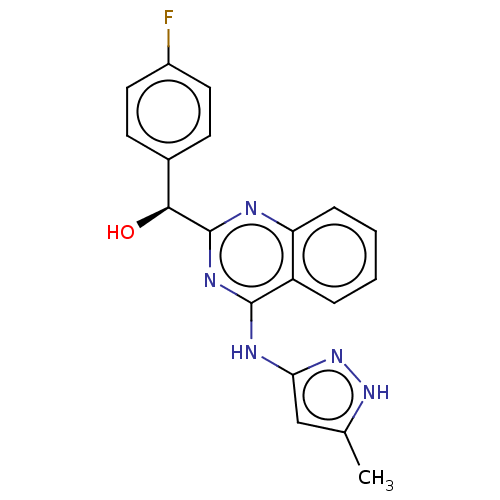
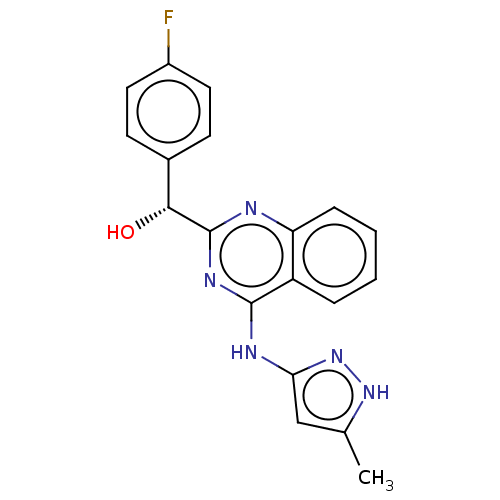
Affinity DataIC50: <310nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: <310nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.05E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.09E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.18E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.47E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.48E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.52E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.59E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.74E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 2.27E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 2.31E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 2.46E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 2.69E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair