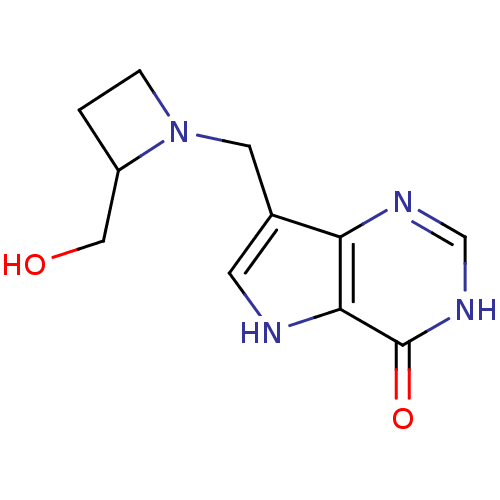
Affinity DataKi: 0.229nM ΔG°: -54.5kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
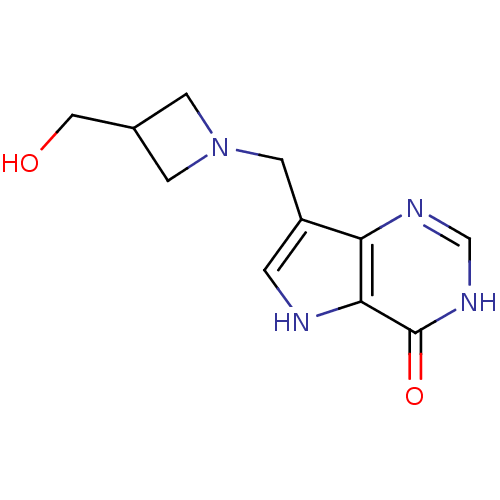
Affinity DataKi: 0.5nM ΔG°: -52.6kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
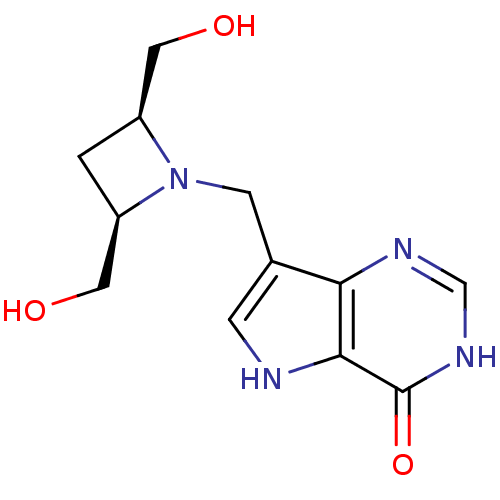
Affinity DataKi: 0.665nM ΔG°: -51.9kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
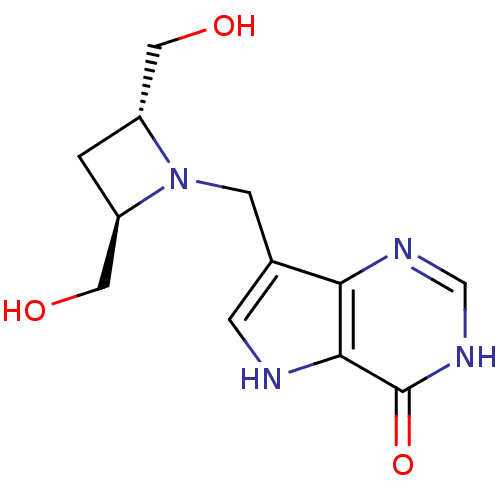
Affinity DataKi: 1nM ΔG°: -50.9kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nM ΔG°: -50.6kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nM ΔG°: -49.4kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nM ΔG°: -49.4kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 4.80nM ΔG°: -47.0kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 6.30nM ΔG°: -46.3kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 12.9nM ΔG°: -44.6kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 16nM ΔG°: -44.0kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 191nM ΔG°: -38.0kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 280nM ΔG°: -37.0kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 360nM ΔG°: -36.4kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 580nM ΔG°: -35.2kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 1.29E+3nM ΔG°: -33.3kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.3kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.3kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair

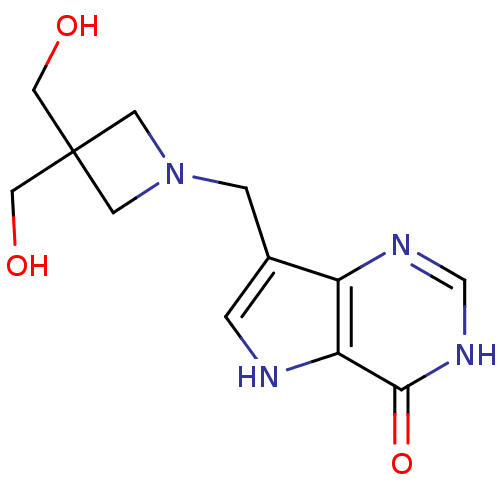
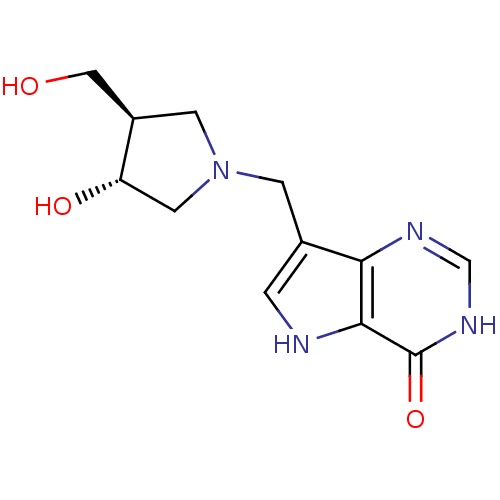
 3D Structure (crystal)
3D Structure (crystal)