Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Oxysterols receptor LXR-alpha [197-447]
Ligand
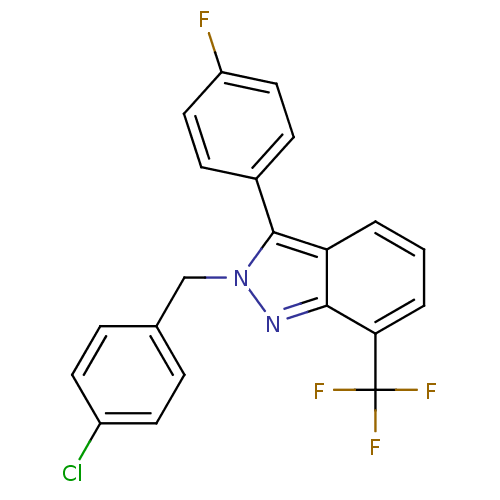
BDBM26070
Substrate
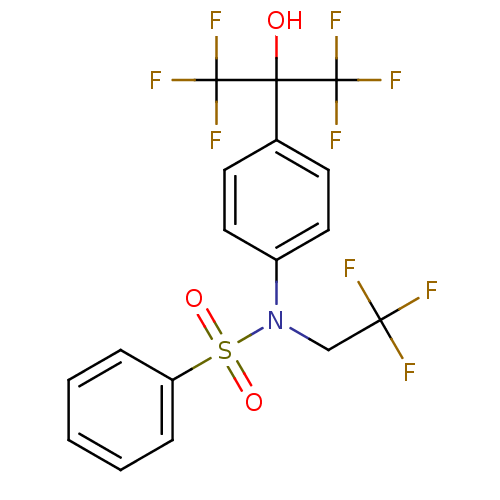
BDBM19993
Meas. Tech.
LXR Binding Assay and hLXR Reporter Assay
IC50
401±n/a nM
EC50
12770±n/a nM
Citation
 Wrobel, J; Steffan†, R; Bowen, SM; Magolda, R; Matelan†, E; Unwalla†, R; Basso, M; Clerin, V; Gardell, SJ; Nambi, P; Quinet, E; Reminick, JI; Vlasuk, GP; Wang, S; Feingold, I; Huselton, C; Bonn, T Indazole-Based Liver X Receptor (LXR) Modulators with Maintained Atherosclerotic Lesion Reduction Activity but Diminished Stimulation of Hepatic Triglyceride Synthesis J Med Chem 51:7161-8 (2008) [PubMed] Article
Wrobel, J; Steffan†, R; Bowen, SM; Magolda, R; Matelan†, E; Unwalla†, R; Basso, M; Clerin, V; Gardell, SJ; Nambi, P; Quinet, E; Reminick, JI; Vlasuk, GP; Wang, S; Feingold, I; Huselton, C; Bonn, T Indazole-Based Liver X Receptor (LXR) Modulators with Maintained Atherosclerotic Lesion Reduction Activity but Diminished Stimulation of Hepatic Triglyceride Synthesis J Med Chem 51:7161-8 (2008) [PubMed] Article More Info.:
Target
Name:
Oxysterols receptor LXR-alpha [197-447]
Synonyms:
LXRA | Liver X Receptor alpha (LXR-alpha) | NR1H3 | NR1H3_HUMAN | Nuclear orphan receptor LXR-alpha | Nuclear receptor subfamily 1 group H member 3 | Oxysterols receptor LXR-alpha
Type:
Receptor
Mol. Mass.:
28986.41
Organism:
Homo sapiens (Human)
Description:
LXR alpha ligand binding domain (amino acid residues 197-447) with an N-terminal biotinylation tag expressed in E.coli, was used for the binding assays.
Residue:
251
Sequence:
SSPPQILPQLSPEQLGMIEKLVAAQQQCNRRSFSDRLRVTPWPMAPDPHSREARQQRFAHFTELAIVSVQEIVDFAKQLPGFLQLSREDQIALLKTSAIEVMLLETSRRYNPGSESITFLKDFSYNREDFAKAGLQVEFINPIFEFSRAMNELQLNDAEFALLIAISIFSADRPNVQDQLQVERLQHTYVEALHAYVSIHHPHDRLMFPRMLMKLVSLRTLSSVHSEQVFALRLQDKKLPPLLSEIWDVHE
Inhibitor
Name:
BDBM26070
Synonyms:
2-[(4-chlorophenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)-2H-indazole | 2-benzyl-3-aryl-7-trifluoromethylindazole, 16
Type:
Small organic molecule
Emp. Form.:
C21H13ClF4N2
Mol. Mass.:
404.788
SMILES:
Fc1ccc(cc1)-c1n(Cc2ccc(Cl)cc2)nc2c(cccc12)C(F)(F)F
Substrate
Name:
BDBM19993
Synonyms:
CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide | T 0901317 | T0901317 | TO-901317 | US10543183, Compound TO901317 | US10669296, Compound TO901317 | US10945978, Compound 1 | [3H]T0901317
Type:
Small organic molecule
Emp. Form.:
C17H12F9NO3S
Mol. Mass.:
481.333
SMILES:
OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F