Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Voltage-dependent N-type calcium channel subunit alpha-1B
Ligand
BDBM50080314
Substrate
n/a
Meas. Tech.
ChEMBL_141329 (CHEMBL752908)
IC50
340±n/a nM
Citation
 Hu, LY; Ryder, TR; Rafferty, MF; Feng, MR; Lotarski, SM; Rock, DM; Sinz, M; Stoehr, SJ; Taylor, CP; Weber, ML; Bowersox, SS; Miljanich, GP; Millerman, E; Wang, YX; Szoke, BG Synthesis of a series of 4-benzyloxyaniline analogues as neuronal N-type calcium channel blockers with improved anticonvulsant and analgesic properties. J Med Chem 42:4239-49 (1999) [PubMed] Article
Hu, LY; Ryder, TR; Rafferty, MF; Feng, MR; Lotarski, SM; Rock, DM; Sinz, M; Stoehr, SJ; Taylor, CP; Weber, ML; Bowersox, SS; Miljanich, GP; Millerman, E; Wang, YX; Szoke, BG Synthesis of a series of 4-benzyloxyaniline analogues as neuronal N-type calcium channel blockers with improved anticonvulsant and analgesic properties. J Med Chem 42:4239-49 (1999) [PubMed] Article More Info.:
Target
Name:
Voltage-dependent N-type calcium channel subunit alpha-1B
Synonyms:
BIII | Brain calcium channel III | CAC1B_HUMAN | CACH5 | CACNA1B | CACNL1A5 | Calcium channel (Type N) | Calcium channel, L type, alpha-1 polypeptide isoform 5 | Voltage-dependent N-type calcium channel subunit alpha-1B | Voltage-dependent N-type calcium channel subunit alpha-1B/Voltage-dependent calcium channel subunit alpha-2/delta-1/Voltage-dependent L-type calcium channel subunit beta-3 | Voltage-gated N-type calcium channel alpha-1B subunit | Voltage-gated N-type calcium channel alpha-1B subunit/Amyloid beta A4 precursor protein-binding family A member 1 | Voltage-gated calcium channel | Voltage-gated calcium channel subunit alpha Cav2.2 | Voltage-gated calcium channel subunit alpha Cav2.2 ((alpha 1B, beta 1b, alpha 2 delta-1) | calcium channel, voltage-dependent, N type, alpha 1B subunit
Type:
Enzyme
Mol. Mass.:
262548.16
Organism:
Homo sapiens (Human)
Description:
Q00975
Residue:
2339
Sequence:
MVRFGDELGGRYGGPGGGERARGGGAGGAGGPGPGGLQPGQRVLYKQSIAQRARTMALYNPIPVKQNCFTVNRSLFVFSEDNVVRKYAKRITEWPPFEYMILATIIANCIVLALEQHLPDGDKTPMSERLDDTEPYFIGIFCFEAGIKIIALGFVFHKGSYLRNGWNVMDFVVVLTGILATAGTDFDLRTLRAVRVLRPLKLVSGIPSLQVVLKSIMKAMVPLLQIGLLLFFAILMFAIIGLEFYMGKFHKACFPNSTDAEPVGDFPCGKEAPARLCEGDTECREYWPGPNFGITNFDNILFAILTVFQCITMEGWTDILYNTNDAAGNTWNWLYFIPLIIIGSFFMLNLVLGVLSGEFAKERERVENRRAFLKLRRQQQIERELNGYLEWIFKAEEVMLAEEDRNAEEKSPLDVLKRAATKKSRNDLIHAEEGEDRFADLCAVGSPFARASLKSGKTESSSYFRRKEKMFRFFIRRMVKAQSFYWVVLCVVALNTLCVAMVHYNQPRRLTTTLYFAEFVFLGLFLTEMSLKMYGLGPRSYFRSSFNCFDFGVIVGSVFEVVWAAIKPGSSFGISVLRALRLLRIFKVTKYWSSLRNLVVSLLNSMKSIISLLFLLFLFIVVFALLGMQLFGGQFNFQDETPTTNFDTFPAAILTVFQILTGEDWNAVMYHGIESQGGVSKGMFSSFYFIVLTLFGNYTLLNVFLAIAVDNLANAQELTKDEEEMEEAANQKLALQKAKEVAEVSPMSAANISIAARQQNSAKARSVWEQRASQLRLQNLRASCEALYSEMDPEERLRFATTRHLRPDMKTHLDRPLVVELGRDGARGPVGGKARPEAAEAPEGVDPPRRHHRHRDKDKTPAAGDQDRAEAPKAESGEPGAREERPRPHRSHSKEAAGPPEARSERGRGPGPEGGRRHHRRGSPEEAAEREPRRHRAHRHQDPSKECAGAKGERRARHRGGPRAGPREAESGEEPARRHRARHKAQPAHEAVEKETTEKEATEKEAEIVEADKEKELRNHQPREPHCDLETSGTVTVGPMHTLPSTCLQKVEEQPEDADNQRNVTRMGSQPPDPNTIVHIPVMLTGPLGEATVVPSGNVDLESQAEGKKEVEADDVMRSGPRPIVPYSSMFCLSPTNLLRRFCHYIVTMRYFEVVILVVIALSSIALAAEDPVRTDSPRNNALKYLDYIFTGVFTFEMVIKMIDLGLLLHPGAYFRDLWNILDFIVVSGALVAFAFSGSKGKDINTIKSLRVLRVLRPLKTIKRLPKLKAVFDCVVNSLKNVLNILIVYMLFMFIFAVIAVQLFKGKFFYCTDESKELERDCRGQYLDYEKEEVEAQPRQWKKYDFHYDNVLWALLTLFTVSTGEGWPMVLKHSVDATYEEQGPSPGYRMELSIFYVVYFVVFPFFFVNIFVALIIITFQEQGDKVMSECSLEKNERACIDFAISAKPLTRYMPQNRQSFQYKTWTFVVSPPFEYFIMAMIALNTVVLMMKFYDAPYEYELMLKCLNIVFTSMFSMECVLKIIAFGVLNYFRDAWNVFDFVTVLGSITDILVTEIAETNNFINLSFLRLFRAARLIKLLRQGYTIRILLWTFVQSFKALPYVCLLIAMLFFIYAIIGMQVFGNIALDDDTSINRHNNFRTFLQALMLLFRSATGEAWHEIMLSCLSNQACDEQANATECGSDFAYFYFVSFIFLCSFLMLNLFVAVIMDNFEYLTRDSSILGPHHLDEFIRVWAEYDPAACGRISYNDMFEMLKHMSPPLGLGKKCPARVAYKRLVRMNMPISNEDMTVHFTSTLMALIRTALEIKLAPAGTKQHQCDAELRKEISVVWANLPQKTLDLLVPPHKPDEMTVGKVYAALMIFDFYKQNKTTRDQMQQAPGGLSQMGPVSLFHPLKATLEQTQPAVLRGARVFLRQKSSTSLSNGGAIQNQESGIKESVSWGTQRTQDAPHEARPPLERGHSTEIPVGRSGALAVDVQMQSITRRGPDGEPQPGLESQGRAASMPRLAAETQPVTDASPMKRSISTLAQRPRGTHLCSTTPDRPPPSQASSHHHHHRCHRRRDRKQRSLEKGPSLSADMDGAPSSAVGPGLPPGEGPTGCRRERERRQERGRSQERRQPSSSSSEKQRFYSCDRFGGREPPKPKPSLSSHPTSPTAGQEPGPHPQGSGSVNGSPLLSTSGASTPGRGGRRQLPQTPLTPRPSITYKTANSSPIHFAGAQTSLPAFSPGRLSRGLSEHNALLQRDPLSQPLAPGSRIGSDPYLGQRLDSEASVHALPEDTLTFEEAVATNSGRSSRTSYVSSLTSQSHPLRRVPNGYHCTLGLSSGGRARHSYHHPDQDHWC
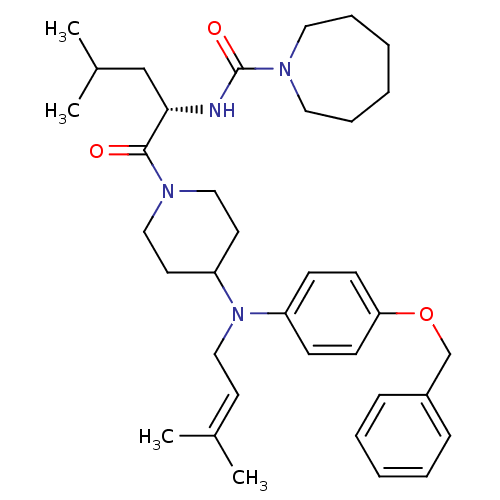
Inhibitor
Name:
BDBM50080314
Synonyms:
Azepane-1-carboxylic acid ((S)-1-{4-[(4-benzyloxy-phenyl)-(3-methyl-but-2-enyl)-amino]-piperidine-1-carbonyl}-3-methyl-butyl)-amide | Azepane-1-carboxylic acid (1-{4-[(4-benzyloxy-phenyl)-(3-methyl-but-2-enyl)-amino]-piperidine-1-carbonyl}-3-methyl-butyl)-amide | CHEMBL311867
Type:
Small organic molecule
Emp. Form.:
C36H52N4O3
Mol. Mass.:
588.8231
SMILES:
[#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6]-1)-[#7](-[#6]\[#6]=[#6](\[#6])-[#6])-c1ccc(-[#8]-[#6]-c2ccccc2)cc1