Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neuropeptide Y receptor type 1
Ligand
BDBM50089046
Substrate
n/a
Meas. Tech.
ChEBML_143672
IC50
>10000±n/a nM
Citation
 Rueeger, H; Rigollier, P; Yamaguchi, Y; Schmidlin, T; Schilling, W; Criscione, L; Whitebread, S; Chiesi, M; Walker, MW; Dhanoa, D; Islam, I; Zhang, J; Gluchowski, C Design, synthesis and SAR of a series of 2-substituted 4-amino-quinazoline neuropeptide Y Y5 receptor antagonists. Bioorg Med Chem Lett 10:1175-9 (2000) [PubMed] Article
Rueeger, H; Rigollier, P; Yamaguchi, Y; Schmidlin, T; Schilling, W; Criscione, L; Whitebread, S; Chiesi, M; Walker, MW; Dhanoa, D; Islam, I; Zhang, J; Gluchowski, C Design, synthesis and SAR of a series of 2-substituted 4-amino-quinazoline neuropeptide Y Y5 receptor antagonists. Bioorg Med Chem Lett 10:1175-9 (2000) [PubMed] Article More Info.:
Target
Name:
Neuropeptide Y receptor type 1
Synonyms:
NPY-Y1 | NPY1-R | NPY1R | NPY1R_HUMAN | NPYR | NPYY1 | neuropeptide Y receptor Y1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
44399.07
Organism:
Homo sapiens (Human)
Description:
NPY-Y1 NPY1R HUMAN::P25929
Residue:
384
Sequence:
MNSTLFSQVENHSVHSNFSEKNAQLLAFENDDCHLPLAMIFTLALAYGAVIILGVSGNLALIIIILKQKEMRNVTNILIVNLSFSDLLVAIMCLPFTFVYTLMDHWVFGEAMCKLNPFVQCVSITVSIFSLVLIAVERHQLIINPRGWRPNNRHAYVGIAVIWVLAVASSLPFLIYQVMTDEPFQNVTLDAYKDKYVCFDQFPSDSHRLSYTTLLLVLQYFGPLCFIFICYFKIYIRLKRRNNMMDKMRDNKYRSSETKRINIMLLSIVVAFAVCWLPLTIFNTVFDWNHQIIATCNHNLLFLLCHLTAMISTCVNPIFYGFLNKNFQRDLQFFFNFCDFRSRDDDYETIAMSTMHTDVSKTSLKQASPVAFKKINNNDDNEKI
Inhibitor
Name:
BDBM50089046
Synonyms:
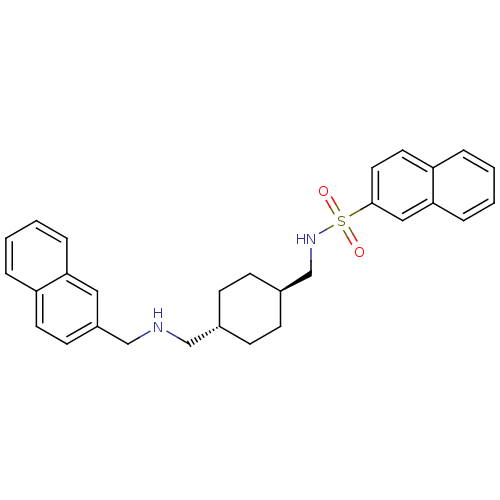
CHEMBL17022 | Naphthalene-2-sulfonic acid (4-{[(naphthalen-2-ylmethyl)-amino]-methyl}-cyclohexylmethyl)-amide
Type:
Small organic molecule
Emp. Form.:
C29H32N2O2S
Mol. Mass.:
472.642
SMILES:
O=S(=O)(NC[C@H]1CC[C@H](CNCc2ccc3ccccc3c2)CC1)c1ccc2ccccc2c1 |wU:8.8,wD:5.4,(14.76,-4.11,;14.78,-5.65,;14.76,-7.18,;13.46,-4.87,;12.12,-5.62,;10.79,-4.85,;10.81,-3.3,;9.47,-2.51,;8.15,-3.28,;6.82,-2.49,;5.48,-3.25,;4.15,-2.49,;2.82,-3.25,;2.82,-4.79,;1.48,-5.55,;.15,-4.78,;-1.18,-5.55,;-2.5,-4.78,;-2.5,-3.23,;-1.18,-2.47,;.15,-3.23,;1.48,-2.47,;8.13,-4.83,;9.45,-5.6,;16.13,-4.9,;16.13,-3.36,;17.48,-2.61,;18.8,-3.4,;20.15,-2.65,;21.47,-3.44,;21.45,-4.99,;20.09,-5.74,;18.78,-4.94,;17.46,-5.68,)|