Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cyclin-K
Ligand
BDBM126500
Substrate
n/a
Meas. Tech.
ChEMBL_1972305 (CHEMBL4605123)
IC50
2.0±n/a nM
Citation
More Info.:
Target
Name:
Cyclin-K
Synonyms:
CCNK | CCNK_HUMAN | CPR4
Type:
PROTEIN
Mol. Mass.:
64254.10
Organism:
Homo sapiens (Human)
Description:
ChEMBL_107901
Residue:
580
Sequence:
MKENKENSSPSVTSANLDHTKPCWYWDKKDLAHTPSQLEGLDPATEARYRREGARFIFDVGTRLGLHYDTLATGIIYFHRFYMFHSFKQFPRYVTGACCLFLAGKVEETPKKCKDIIKTARSLLNDVQFGQFGDDPKEEVMVLERILLQTIKFDLQVEHPYQFLLKYAKQLKGDKNKIQKLVQMAWTFVNDSLCTTLSLQWEPEIIAVAVMYLAGRLCKFEIQEWTSKPMYRRWWEQFVQDVPVDVLEDICHQILDLYSQGKQQMPHHTPHQLQQPPSLQPTPQVPQVQQSQPSQSSEPSQPQQKDPQQPAQQQQPAQQPKKPSPQPSSPRQVKRAVVVSPKEENKAAEPPPPKIPKIETTHPPLPPAHPPPDRKPPLAAALGEAEPPGPVDATDLPKVQIPPPAHPAPVHQPPPLPHRPPPPPPSSYMTGMSTTSSYMSGEGYQSLQSMMKTEGPSYGALPPAYGPPAHLPYHPHVYPPNPPPPPVPPPPASFPPPAIPPPTPGYPPPPPTYNPNFPPPPPRLPPTHAVPPHPPPGLGLPPASYPPPAVPPGGQPPVPPPIPPPGMPPVGGLGRAAWMR
Inhibitor
Name:
BDBM126500
Synonyms:
US11591322, Compound NVP-2 | US8778951, 310
Type:
Small organic molecule
Emp. Form.:
C27H37ClN6O2
Mol. Mass.:
513.075
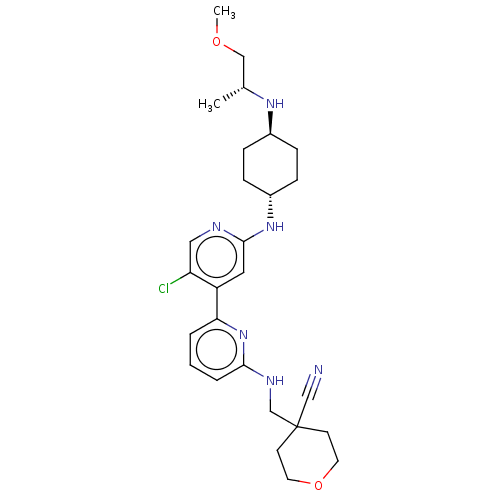
SMILES:
COC[C@@H](C)N[C@H]1CC[C@@H](CC1)Nc1cc(c(Cl)cn1)-c1cccc(NCC2(CCOCC2)C#N)n1 |r,wU:9.12,wD:6.5,3.3,(7.56,1.68,;6.23,2.45,;4.9,1.68,;3.56,2.45,;3.56,3.99,;2.23,1.68,;.89,2.45,;.89,3.99,;-.44,4.76,;-1.77,3.99,;-1.77,2.45,;-.44,1.68,;-3.11,4.76,;-4.44,3.99,;-4.44,2.45,;-5.77,1.68,;-7.11,2.45,;-8.44,1.68,;-7.11,3.99,;-5.77,4.76,;-5.77,.14,;-7.11,-.63,;-7.11,-2.17,;-5.77,-2.94,;-4.44,-2.17,;-3.11,-2.94,;-1.77,-2.17,;-.44,-2.94,;-.44,-4.48,;.89,-5.25,;2.23,-4.48,;2.23,-2.94,;.89,-2.17,;-.44,-1.4,;-.44,.14,;-4.44,-.63,)|