Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prothrombin
Ligand
BDBM50125017
Substrate
n/a
Meas. Tech.
ChEMBL_208906 (CHEMBL814961)
Ki
0.650000±n/a nM
Citation
 Su, T; Yang, H; Volkots, D; Woolfrey, J; Dam, S; Wong, P; Sinha, U; Scarborough, RM; Zhu, BY Design, synthesis, and structure-activity relationships of substituted piperazinone-based transition state factor Xa inhibitors. Bioorg Med Chem Lett 13:729-32 (2003) [PubMed] Article
Su, T; Yang, H; Volkots, D; Woolfrey, J; Dam, S; Wong, P; Sinha, U; Scarborough, RM; Zhu, BY Design, synthesis, and structure-activity relationships of substituted piperazinone-based transition state factor Xa inhibitors. Bioorg Med Chem Lett 13:729-32 (2003) [PubMed] Article More Info.:
Target
Name:
Prothrombin
Synonyms:
Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain
Type:
Protein
Mol. Mass.:
70029.57
Organism:
Homo sapiens (Human)
Description:
P00734
Residue:
622
Sequence:
MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLERECVEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHVNITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQECSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASAQAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETGDGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYIDGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTENDLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHPVCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDSTRIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKYGFYTHVFRLKKWIQKVIDQFGE
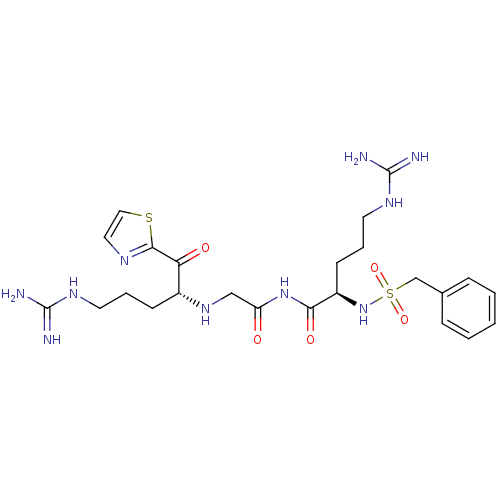
Inhibitor
Name:
BDBM50125017
Synonyms:
CHEMBL350976 | N-((R)-5-Guanidino-2-phenylmethanesulfonylamino-pentanoyl)-2-[(R)-4-guanidino-1-(thiazole-2-carbonyl)-butylamino]-acetamide
Type:
Small organic molecule
Emp. Form.:
C24H36N10O5S2
Mol. Mass.:
608.737
SMILES:
NC(=N)NCCC[C@@H](NCC(=O)NC(=O)[C@@H](CCCNC(N)=N)NS(=O)(=O)Cc1ccccc1)C(=O)c1nccs1