Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prostaglandin D2 receptor
Ligand
BDBM50161746
Substrate
n/a
Meas. Tech.
ChEMBL_303004 (CHEMBL830243)
Ki
>10000±n/a nM
Citation
 Ulven, T; Kostenis, E Minor structural modifications convert the dual TP/CRTH2 antagonist ramatroban into a highly selective and potent CRTH2 antagonist. J Med Chem 48:897-900 (2005) [PubMed] Article
Ulven, T; Kostenis, E Minor structural modifications convert the dual TP/CRTH2 antagonist ramatroban into a highly selective and potent CRTH2 antagonist. J Med Chem 48:897-900 (2005) [PubMed] Article More Info.:
Target
Name:
Prostaglandin D2 receptor
Synonyms:
PD2R_HUMAN | PTGDR | Prostaglandin D2 | Prostaglandin D2 receptor | Prostanoid DP receptor
Type:
Enzyme
Mol. Mass.:
40288.87
Organism:
Homo sapiens (Human)
Description:
Q13258
Residue:
359
Sequence:
MKSPFYRCQNTTSVEKGNSAVMGGVLFSTGLLGNLLALGLLARSGLGWCSRRPLRPLPSVFYMLVCGLTVTDLLGKCLLSPVVLAAYAQNRSLRVLAPALDNSLCQAFAFFMSFFGLSSTLQLLAMALECWLSLGHPFFYRRHITLRLGALVAPVVSAFSLAFCALPFMGFGKFVQYCPGTWCFIQMVHEEGSLSVLGYSVLYSSLMALLVLATVLCNLGAMRNLYAMHRRLQRHPRSCTRDCAEPRADGREASPQPLEELDHLLLLALMTVLFTMCSLPVIYRAYYGAFKDVKEKNRTSEEAEDLRALRFLSVISIVDPWIFIIFRSPVFRIFFHKIFIRPLRYRSRCSNSTNMESSL
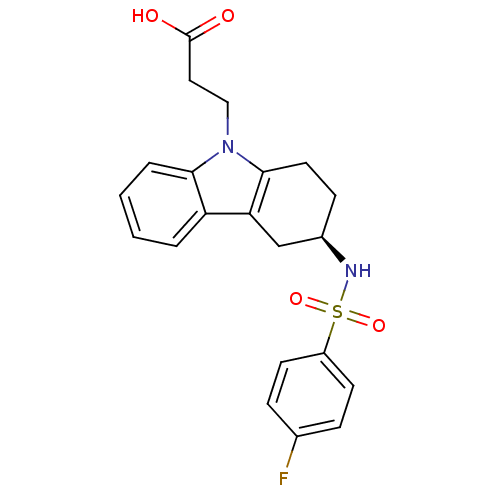
Inhibitor
Name:
BDBM50161746
Synonyms:
(R)-3-(3-(4-fluorophenylsulfonamido)-1,2,3,4-tetrahydrocarbazol-9-yl)propanoic acid | 3-[(R)-3-(4-Fluoro-benzenesulfonylamino)-1,2,3,4-tetrahydro-carbazol-9-yl]-propionic acid | CHEMBL361812 | RAMATROBAN
Type:
Small organic molecule
Emp. Form.:
C21H21FN2O4S
Mol. Mass.:
416.466
SMILES:
OC(=O)CCn1c2CC[C@H](Cc2c2ccccc12)NS(=O)(=O)c1ccc(F)cc1