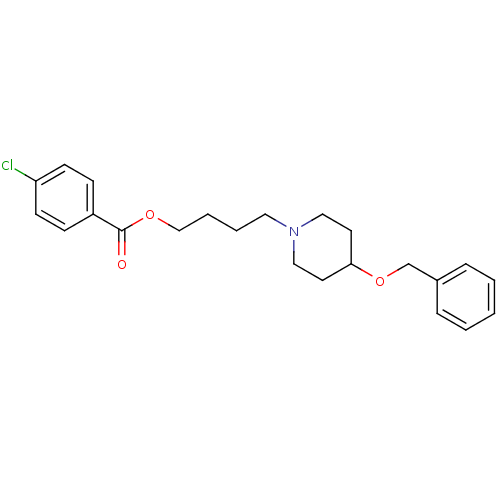
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cholinesterase
Ligand
BDBM50219244
Substrate
n/a
Meas. Tech.
ChEMBL_437418 (CHEMBL906816)
IC50
3520±n/a nM
Citation
 Kwon, YE; Park, JY; No, KT; Shin, JH; Lee, SK; Eun, JS; Yang, JH; Shin, TY; Kim, DK; Chae, BS; Leem, JY; Kim, KH Synthesis, in vitro assay, and molecular modeling of new piperidine derivatives having dual inhibitory potency against acetylcholinesterase and Abeta1-42 aggregation for Alzheimer's disease therapeutics. Bioorg Med Chem 15:6596-607 (2007) [PubMed] Article
Kwon, YE; Park, JY; No, KT; Shin, JH; Lee, SK; Eun, JS; Yang, JH; Shin, TY; Kim, DK; Chae, BS; Leem, JY; Kim, KH Synthesis, in vitro assay, and molecular modeling of new piperidine derivatives having dual inhibitory potency against acetylcholinesterase and Abeta1-42 aggregation for Alzheimer's disease therapeutics. Bioorg Med Chem 15:6596-607 (2007) [PubMed] Article More Info.:
Target
Name:
Cholinesterase
Synonyms:
Acylcholine acylhydrolase | BCHE | Butyrylcholine esterase (BChE) | Butyrylcholinesterase (BChE) | Butyrylcholinesterase (BuChE) | CHE1 | CHLE_HUMAN | Choline esterase II | Cholinesterases | Cholinesterases; ACHE & BCHE | Pseudocholinesterase
Type:
Homotetramer
Mol. Mass.:
68422.27
Organism:
Homo sapiens (Human)
Description:
P06276
Residue:
602
Sequence:
MHSKVTIICIRFLFWFLLLCMLIGKSHTEDDIIIATKNGKVRGMNLTVFGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSDIWNATKYANSCCQNIDQSFPGFHGSEMWNPNTDLSEDCLYLNVWIPAPKPKNATVLIWIYGGGFQTGTSSLHVYDGKFLARVERVIVVSMNYRVGALGFLALPGNPEAPGNMGLFDQQLALQWVQKNIAAFGGNPKSVTLFGESAGAASVSLHLLSPGSHSLFTRAILQSGSFNAPWAVTSLYEARNRTLNLAKLTGCSRENETEIIKCLRNKDPQEILLNEAFVVPYGTPLSVNFGPTVDGDFLTDMPDILLELGQFKKTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPGVSEFGKESILFHYTDWVDDQRPENYREALGDVVGDYNFICPALEFTKKFSEWGNNAFFYYFEHRSSKLPWPEWMGVMHGYEIEFVFGLPLERRDNYTKAEEILSRSIVKRWANFAKYGNPNETQNNSTSWPVFKSTEQKYLTLNTESTRIMTKLRAQQCRFWTSFFPKVLEMTGNIDEAEWEWKAGFHRWNNYMMDWKNQFNDYTSKKESCVGL