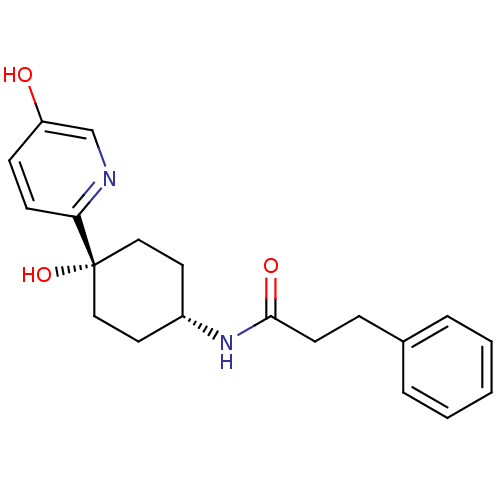
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Potassium voltage-gated channel subfamily H member 2
Ligand
BDBM50220714
Substrate
n/a
Meas. Tech.
ChEMBL_449357 (CHEMBL899624)
IC50
>30000±n/a nM
Citation
 Kawai, M; Nakamura, H; Sakurada, I; Shimokawa, H; Tanaka, H; Matsumizu, M; Ando, K; Hattori, K; Ohta, A; Nukui, S; Omura, A; Kawamura, M Discovery of novel and orally active NR2B-selective N-methyl-D-aspartate (NMDA) antagonists, pyridinol derivatives with reduced HERG binding affinity. Bioorg Med Chem Lett 17:5533-6 (2007) [PubMed] Article
Kawai, M; Nakamura, H; Sakurada, I; Shimokawa, H; Tanaka, H; Matsumizu, M; Ando, K; Hattori, K; Ohta, A; Nukui, S; Omura, A; Kawamura, M Discovery of novel and orally active NR2B-selective N-methyl-D-aspartate (NMDA) antagonists, pyridinol derivatives with reduced HERG binding affinity. Bioorg Med Chem Lett 17:5533-6 (2007) [PubMed] Article More Info.:
Target
Name:
Potassium voltage-gated channel subfamily H member 2
Synonyms:
1,3-beta-glucan synthase component GLS2 | Cytochrome P450 3A4 | ERG | ERG1 | Eag-related protein 1 | Ether a-go-go related gene potassium channel (hERG) | Ether-a-go-go-related gene (HERG) | Ether-a-go-go-related gene potassium channel (hERG) | Ether-a-go-go-related gene potassium channel 1 | Ether-a-go-go-related gene potassium channel 1 (HERG) | Ether-a-go-go-related gene potassium channel 1 (hERG1) | Ether-a-go-go-related protein (hERG) | Ether-a-go-go-related protein 1 | Ether-a-go-go-related protein 1 (HERG) | H-ERG | HERG | KCNH2 | KCNH2_HUMAN | Potassium voltage-gated channel subfamily H member 2 (hERG) | Transcriptional regulator ERG | Voltage-gated potassium channel subunit Kv11.1 | eag homolog | hERG Potassium Channel 1 | putative potassium channel subunit
Type:
Multi-pass membrane protein
Mol. Mass.:
126672.65
Organism:
Homo sapiens (Human)
Description:
Q12809
Residue:
1159
Sequence:
MPVRRGHVAPQNTFLDTIIRKFEGQSRKFIIANARVENCAVIYCNDGFCELCGYSRAEVMQRPCTCDFLHGPRTQRRAAAQIAQALLGAEERKVEIAFYRKDGSCFLCLVDVVPVKNEDGAVIMFILNFEVVMEKDMVGSPAHDTNHRGPPTSWLAPGRAKTFRLKLPALLALTARESSVRSGGAGGAGAPGAVVVDVDLTPAAPSSESLALDEVTAMDNHVAGLGPAEERRALVGPGSPPRSAPGQLPSPRAHSLNPDASGSSCSLARTRSRESCASVRRASSADDIEAMRAGVLPPPPRHASTGAMHPLRSGLLNSTSDSDLVRYRTISKIPQITLNFVDLKGDPFLASPTSDREIIAPKIKERTHNVTEKVTQVLSLGADVLPEYKLQAPRIHRWTILHYSPFKAVWDWLILLLVIYTAVFTPYSAAFLLKETEEGPPATECGYACQPLAVVDLIVDIMFIVDILINFRTTYVNANEEVVSHPGRIAVHYFKGWFLIDMVAAIPFDLLIFGSGSEELIGLLKTARLLRLVRVARKLDRYSEYGAAVLFLLMCTFALIAHWLACIWYAIGNMEQPHMDSRIGWLHNLGDQIGKPYNSSGLGGPSIKDKYVTALYFTFSSLTSVGFGNVSPNTNSEKIFSICVMLIGSLMYASIFGNVSAIIQRLYSGTARYHTQMLRVREFIRFHQIPNPLRQRLEEYFQHAWSYTNGIDMNAVLKGFPECLQADICLHLNRSLLQHCKPFRGATKGCLRALAMKFKTTHAPPGDTLVHAGDLLTALYFISRGSIEILRGDVVVAILGKNDIFGEPLNLYARPGKSNGDVRALTYCDLHKIHRDDLLEVLDMYPEFSDHFWSSLEITFNLRDTNMIPGSPGSTELEGGFSRQRKRKLSFRRRTDKDTEQPGEVSALGPGRAGAGPSSRGRPGGPWGESPSSGPSSPESSEDEGPGRSSSPLRLVPFSSPRPPGEPPGGEPLMEDCEKSSDTCNPLSGAFSGVSNIFSFWGDSRGRQYQELPRCPAPTPSLLNIPLSSPGRRPRGDVESRLDALQRQLNRLETRLSADMATVLQLLQRQMTLVPPAYSAVTTPGPGPTSTSPLLPVSPLPTLTLDSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPGQLGALTSQPLHRHGSDPGS
Inhibitor
Name:
BDBM50220714
Synonyms:
CHEMBL237751 | N-((1s,4s)-4-hydroxy-4-(5-hydroxypyridin-2-yl)cyclohexyl)-3-phenylpropanamide
Type:
Small organic molecule
Emp. Form.:
C20H24N2O3
Mol. Mass.:
340.4162
SMILES:
Oc1ccc(nc1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:7.8,11.15,(-10.78,-55,;-9.45,-54.23,;-9.45,-52.68,;-8.13,-51.91,;-6.79,-52.68,;-6.78,-54.21,;-8.1,-54.99,;-5.45,-51.91,;-5.46,-53.44,;-5.45,-50.37,;-4.13,-49.58,;-2.79,-50.37,;-2.79,-51.91,;-4.13,-52.68,;-1.45,-49.6,;-.12,-50.37,;-.12,-51.91,;1.23,-49.61,;2.55,-50.39,;3.89,-49.63,;5.21,-50.4,;6.55,-49.65,;6.56,-48.11,;5.24,-47.33,;3.9,-48.09,)|