Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50260759
Substrate
n/a
Meas. Tech.
ChEMBL_495153 (CHEMBL1008932)
IC50
36000±n/a nM
Citation
 Chen, C; Wu, D; Guo, Z; Xie, Q; Reinhart, GJ; Madan, A; Wen, J; Chen, T; Huang, CQ; Chen, M; Chen, Y; Tucci, FC; Rowbottom, M; Pontillo, J; Zhu, YF; Wade, W; Saunders, J; Bozigian, H; Struthers, RS Discovery of sodium R-(+)-4-{2-[5-(2-fluoro-3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino}butyrate (elagolix), a potent and orally available nonpeptide antagonist of the human gonadotropin-releasing hormone receptor. J Med Chem 51:7478-85 (2009) [PubMed] Article
Chen, C; Wu, D; Guo, Z; Xie, Q; Reinhart, GJ; Madan, A; Wen, J; Chen, T; Huang, CQ; Chen, M; Chen, Y; Tucci, FC; Rowbottom, M; Pontillo, J; Zhu, YF; Wade, W; Saunders, J; Bozigian, H; Struthers, RS Discovery of sodium R-(+)-4-{2-[5-(2-fluoro-3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino}butyrate (elagolix), a potent and orally available nonpeptide antagonist of the human gonadotropin-releasing hormone receptor. J Med Chem 51:7478-85 (2009) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
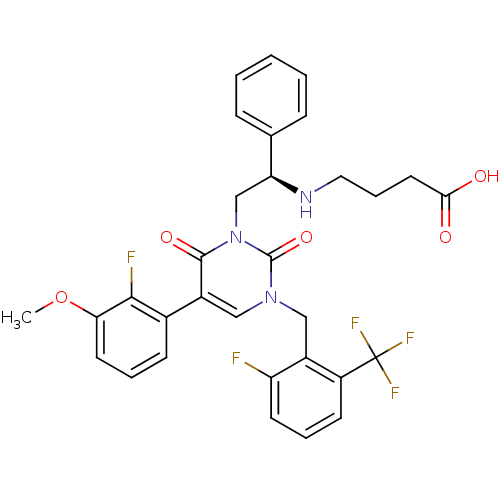
BDBM50260759
Synonyms:
(R)-4-(2-(3-(2-fluoro-6-(trifluoromethyl)benzyl)-5-(2-fluoro-3-methoxyphenyl)-2,6-dioxo-2,3-dihydropyrimidin-1(6H)-yl)-1-phenylethylamino)butanoic acid | (R)-4-{2-[5-(2-Fluoro-3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino}butyric Acid | CHEMBL509075
Type:
Small organic molecule
Emp. Form.:
C31H28F5N3O5
Mol. Mass.:
617.5631
SMILES:
COc1cccc(c1F)-c1cn(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](NCCCC(O)=O)c2ccccc2)c1=O |r|