Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Sphingosine 1-phosphate receptor 3
Ligand
BDBM50158348
Substrate
n/a
Meas. Tech.
ChEMBL_632509 (CHEMBL1111157)
EC50
0.70±n/a nM
Citation
 Bolli, MH; Abele, S; Binkert, C; Bravo, R; Buchmann, S; Bur, D; Gatfield, J; Hess, P; Kohl, C; Mangold, C; Mathys, B; Menyhart, K; Müller, C; Nayler, O; Scherz, M; Schmidt, G; Sippel, V; Steiner, B; Strasser, D; Treiber, A; Weller, T 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem 53:4198-211 (2010) [PubMed] Article
Bolli, MH; Abele, S; Binkert, C; Bravo, R; Buchmann, S; Bur, D; Gatfield, J; Hess, P; Kohl, C; Mangold, C; Mathys, B; Menyhart, K; Müller, C; Nayler, O; Scherz, M; Schmidt, G; Sippel, V; Steiner, B; Strasser, D; Treiber, A; Weller, T 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem 53:4198-211 (2010) [PubMed] Article More Info.:
Target
Name:
Sphingosine 1-phosphate receptor 3
Synonyms:
C9orf108 | C9orf47 | EDG3 | Endothelial differentiation G-protein coupled receptor 3 | S1P receptor 3 | S1P receptor Edg-3 | S1P3 | S1PR3 | S1PR3_HUMAN | Sphingosine 1-phosphate receptor | Sphingosine 1-phosphate receptor 3 (S1P3) | Sphingosine 1-phosphate receptor Edg-3
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
42278.13
Organism:
Homo sapiens (Human)
Description:
Q99500
Residue:
378
Sequence:
MATALPPRLQPVRGNETLREHYQYVGKLAGRLKEASEGSTLTTVLFLVICSFIVLENLMVLIAIWKNNKFHNRMYFFIGNLALCDLLAGIAYKVNILMSGKKTFSLSPTVWFLREGSMFVALGASTCSLLAIAIERHLTMIKMRPYDANKRHRVFLLIGMCWLIAFTLGALPILGWNCLHNLPDCSTILPLYSKKYIAFCISIFTAILVTIVILYARIYFLVKSSSRKVANHNNSERSMALLRTVVIVVSVFIACWSPLFILFLIDVACRVQACPILFKAQWFIVLAVLNSAMNPVIYTLASKEMRRAFFRLVCNCLVRGRGARASPIQPALDPSRSKSSSSNNSSHSPKVKEDLPHTAPSSCIMDKNAALQNGIFCN
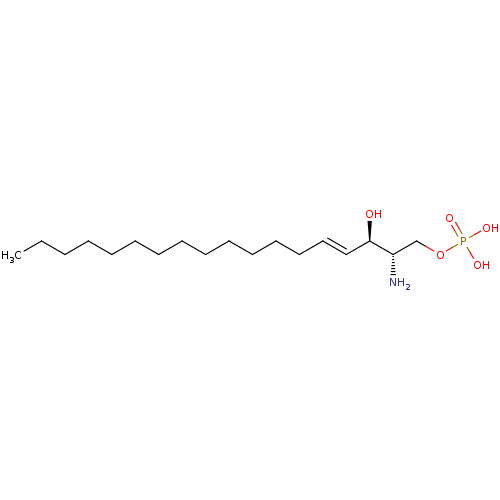
Inhibitor
Name:
BDBM50158348
Synonyms:
(2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihydrogen phosphate | (2S,3R,4E)-2-amino-4-octadecene-1,3-diol 1-(dihydrogen phosphate) | CHEMBL225155 | US10676467, Compound S1P | US11584726, Example S1P | sphingosine 1-phosphate | sphingosine-1-phosphate
Type:
Small organic molecule
Emp. Form.:
C18H38NO5P
Mol. Mass.:
379.4718
SMILES:
CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r|