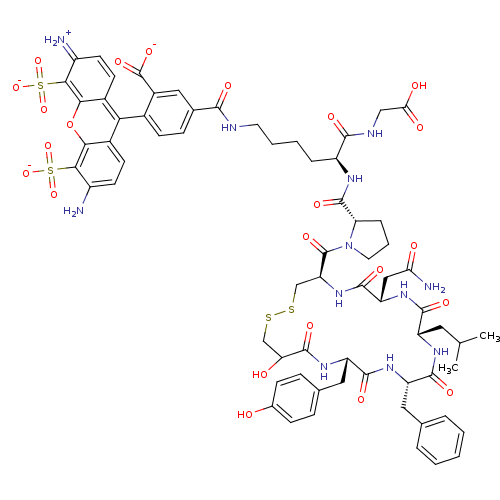
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Vasopressin V2 receptor
Ligand
BDBM50342035
Substrate
n/a
Meas. Tech.
ChEMBL_743721 (CHEMBL1767535)
Ki
>100000±n/a nM
Citation
 Corbani, M; Trueba, M; Stoev, S; Murat, B; Mion, J; Boulay, V; Guillon, G; Manning, M Design, synthesis, and pharmacological characterization of fluorescent peptides for imaging human V1b vasopressin or oxytocin receptors. J Med Chem 54:2864-77 (2011) [PubMed] Article
Corbani, M; Trueba, M; Stoev, S; Murat, B; Mion, J; Boulay, V; Guillon, G; Manning, M Design, synthesis, and pharmacological characterization of fluorescent peptides for imaging human V1b vasopressin or oxytocin receptors. J Med Chem 54:2864-77 (2011) [PubMed] Article More Info.:
Target
Name:
Vasopressin V2 receptor
Synonyms:
ADHR | AVPR V2 | AVPR2 | Antidiuretic hormone receptor | DIR | DIR3 | Renal-type arginine vasopressin receptor | V2R | V2R_HUMAN | VASOPRESSIN V2 | Vasopressin V2 receptor | Vasopressin receptor
Type:
Receptor
Mol. Mass.:
40295.28
Organism:
Homo sapiens (Human)
Description:
P30518
Residue:
371
Sequence:
MLMASTTSAVPGHPSLPSLPSNSSQERPLDTRDPLLARAELALLSIVFVAVALSNGLVLAALARRGRRGHWAPIHVFIGHLCLADLAVALFQVLPQLAWKATDRFRGPDALCRAVKYLQMVGMYASSYMILAMTLDRHRAICRPMLAYRHGSGAHWNRPVLVAWAFSLLLSLPQLFIFAQRNVEGGSGVTDCWACFAEPWGRRTYVTWIALMVFVAPTLGIAACQVLIFREIHASLVPGPSERPGGRRRGRRTGSPGEGAHVSAAVAKTVRMTLVIVVVYVLCWAPFFLVQLWAAWDPEAPLEGAPFVLLMLLASLNSCTNPWIYASFSSSVSSELRSLLCCARGRTPPSLGPQDESCTTASSSLAKDTSS
Inhibitor
Name:
BDBM50342035
Synonyms:
CHEMBL1765670 | triethylamine hemi(2-((2S)-2-((2S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-13-benzyl-19-hydroxy-16-(4-hydroxybenzyl)-10-isobutyl-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carbonyl)pyrrolidine-2-carboxamido)-6-(2-(6-amino-3-imino-4,5-disulfonato-3H-xanthen-9-yl)-5-((2,3,5,6-tetrafluorophenoxy)carbonyl)benzamido)hexanamido)acetate)
Type:
Small organic molecule
Emp. Form.:
C68H76N12O23S4
Mol. Mass.:
1557.659
SMILES:
CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)C(O)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc(c(c1)C([O-])=O)-c1c2ccc(N)c(c2oc2c(c(=[NH2+])ccc12)S([O-])(=O)=O)S([O-])(=O)=O)C(=O)NCC(O)=O |r,wU:19.20,4.3,55.59,wD:8.8,41.43,59.62,37.51,(14.23,7.82,;14.23,6.28,;15.57,5.51,;12.88,5.51,;12.88,3.97,;11.55,3.19,;10.21,3.97,;10.21,5.51,;8.88,3.19,;8.88,1.65,;10.21,.87,;11.55,1.65,;12.88,.87,;12.88,-.67,;11.55,-1.45,;10.21,-.67,;7.53,3.97,;6.2,3.19,;6.2,1.65,;4.86,3.97,;4.86,5.51,;6.2,6.28,;7.53,5.51,;8.88,6.28,;8.88,7.82,;10.21,8.62,;7.53,8.62,;6.2,7.82,;3.52,3.19,;2.19,2.42,;.86,3.19,;2.19,.88,;3.66,.42,;.86,-1.06,;12.23,-4.85,;22.09,-.55,;20.94,.9,;20.91,2.44,;19.57,3.19,;18.24,3.97,;18.24,5.51,;16.9,3.19,;16.82,1.65,;18.12,.81,;19.49,1.51,;18.04,-.73,;15.57,3.97,;14.23,3.19,;14.23,1.65,;22.24,3.23,;22.22,4.76,;23.59,2.47,;24.05,4.06,;25.71,4.12,;26.27,2.57,;24.92,1.83,;24.97,.32,;23.63,-.41,;26.27,-.48,;26.23,-2.01,;24.89,-2.73,;24.85,-4.26,;23.51,-4.98,;23.47,-6.51,;22.13,-7.24,;20.77,-6.51,;20.73,-4.97,;19.46,-7.32,;19.5,-8.86,;18.19,-9.66,;16.83,-8.92,;16.79,-7.39,;18.1,-6.59,;15.98,-6.09,;16.72,-4.73,;14.44,-6.13,;15.52,-9.73,;15.56,-11.27,;16.92,-11.99,;16.96,-13.53,;15.64,-14.34,;15.68,-15.88,;14.29,-13.61,;14.24,-12.07,;12.88,-11.34,;12.85,-9.79,;11.5,-9.07,;11.45,-7.53,;10.1,-6.8,;12.77,-6.72,;14.12,-7.46,;14.16,-8.99,;10.19,-9.88,;8.83,-9.15,;9.46,-11.23,;11,-11.19,;12.98,-14.42,;11.62,-13.69,;12.25,-15.78,;13.79,-15.73,;27.53,-2.8,;28.87,-2.08,;27.49,-4.33,;28.79,-5.13,;28.75,-6.65,;30.05,-7.45,;27.41,-7.38,)|