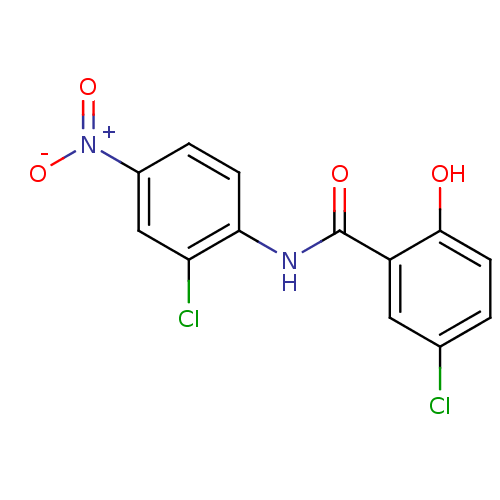
BDBM11242 5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide::med.21724, Compound 76::niclosamide
SMILES c1cc(c(cc1[N+](=O)[O-])Cl)NC(=O)c2cc(ccc2O)Cl
InChI Key InChIKey=RJMUSRYZPJIFPJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 31 hits for monomerid = 11242
Found 31 hits for monomerid = 11242
Affinity DataIC50: 28nMAssay Description:Inhibition of YFP tagged ANO1 (unknown origin) expressed in FRT cells incubated for 24 hrs by fluorescence plate reader assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Human)
The First Affiliated Hospital of Wenzhou Medical University
Curated by ChEMBL
The First Affiliated Hospital of Wenzhou Medical University
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of STAT3 phosphorylation in human MDA-MB-231 cells by sandwich ELISAMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Human)
The First Affiliated Hospital of Wenzhou Medical University
Curated by ChEMBL
The First Affiliated Hospital of Wenzhou Medical University
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of STAT3 in human HeLa cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Ten-point DRCs were generated for each drug. Vero cells were seeded at 1.2 × 104 cells per well in DMEM, supplemented with 2% FBS and 1× ...More data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Inhibition of SARS-CoV-2 main proteaseMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Human)
The First Affiliated Hospital of Wenzhou Medical University
Curated by ChEMBL
The First Affiliated Hospital of Wenzhou Medical University
Curated by ChEMBL
Affinity DataIC50: 1.93E+3nMAssay Description:Inhibition of human recombinant STAT3 assessed as reduction in DNA binding activity with HepG2 nuclear extract incubated for 1 hr by ELISA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of JAK2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Src kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.05E+4nMAssay Description:Inhibition of EGFR after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.05E+4nMAssay Description:Inhibition of Src kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.31E+4nMAssay Description:Inhibition of FGFR-1 after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.31E+4nMAssay Description:Inhibition of VEGFR2 after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.38E+4nMAssay Description:Inhibition of LYN after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.78E+4nMAssay Description:Inhibition of KIT after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of SARS Co-V 3CL proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMpH: 7.0 T: 2°CAssay Description:The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t...More data for this Ligand-Target Pair
Affinity DataIC50: 5.41E+4nMAssay Description:Inhibition of Aurora-A after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.94E+4nMAssay Description:Inhibition of Akt1 after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.78E+4nMAssay Description:Inhibition of TIE2 after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.75E+4nMAssay Description:Inhibition of GSK3-beta after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.39E+4nMAssay Description:Inhibition of PDK1 after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of JNK1 after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PDGFR-beta after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Abl1 after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PKC-alpha after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of B-Raf after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Erk2 after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Flt3 after 60 mins by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of JAK2 after 60 mins by radiometric assayMore data for this Ligand-Target Pair