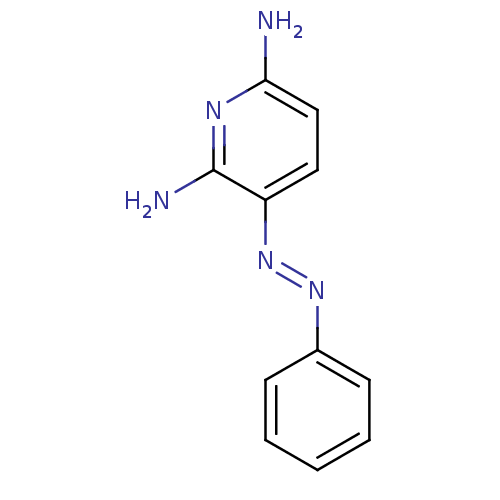
BDBM50420356 NC 150::PHENAZOPYRIDINE HYDROCHLORIDE
SMILES Nc1ccc(\N=N\c2ccccc2)c(N)n1
InChI Key InChIKey=QPFYXYFORQJZEC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50420356
Found 3 hits for monomerid = 50420356
Affinity DataIC50: 5.43E+4nMAssay Description:Inhibition of snake venom BaP1 using Abz-Ala-Gly-Leu-Ala-Nba as substrate incubated for 30 mins prior to substrate addition by fluorescence spectroph...More data for this Ligand-Target Pair
Affinity DataKi: 7.00E+4nMAssay Description:Competitive inhibition of recombinant human SARM1 TIR domain (561 to 724 residues) expressed in Escherichia coli C43 (DE3) cells lysates assessed as ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+5nMAssay Description:Inhibition of recombinant human SARM1 TIR domain (561 to 724 residues) expressed in Escherichia coli C43 (DE3) cells lysates using ENAD as substrate ...More data for this Ligand-Target Pair