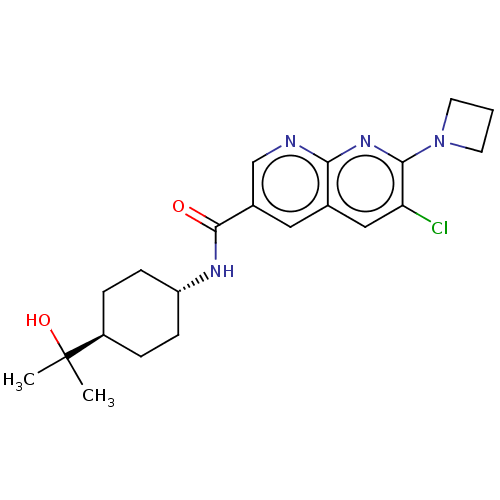
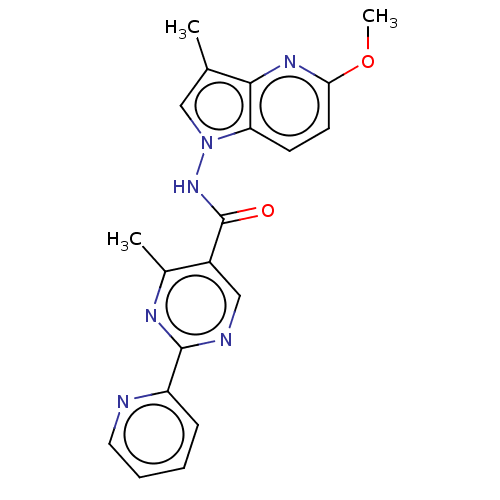
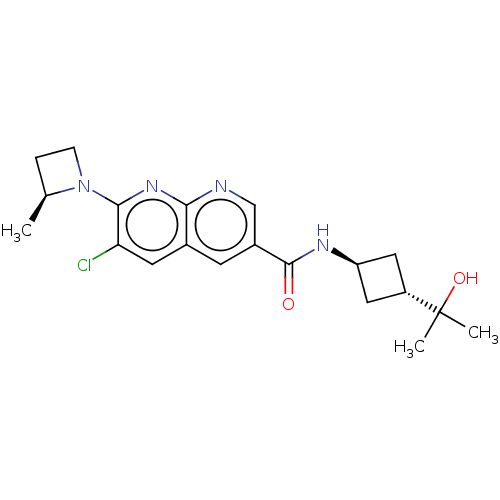
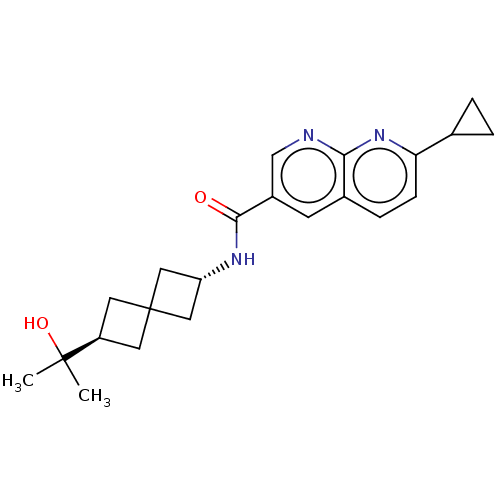
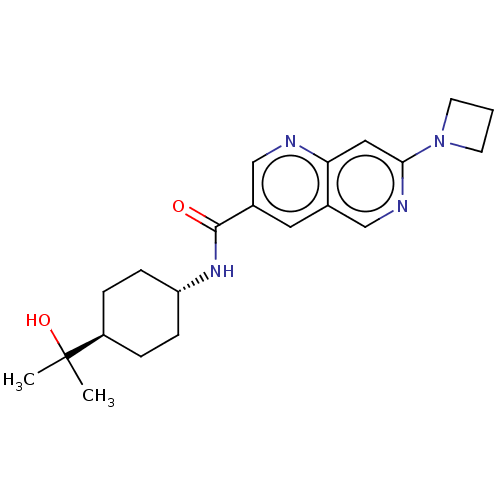
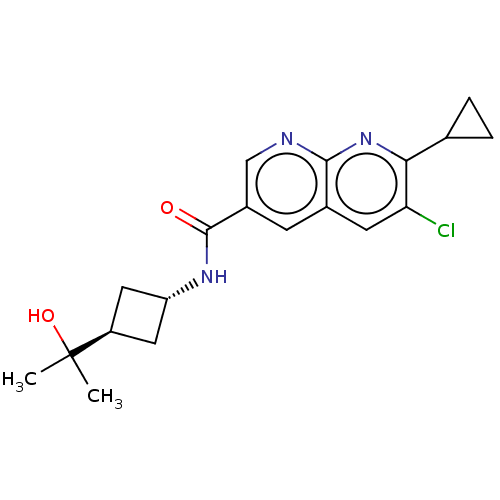
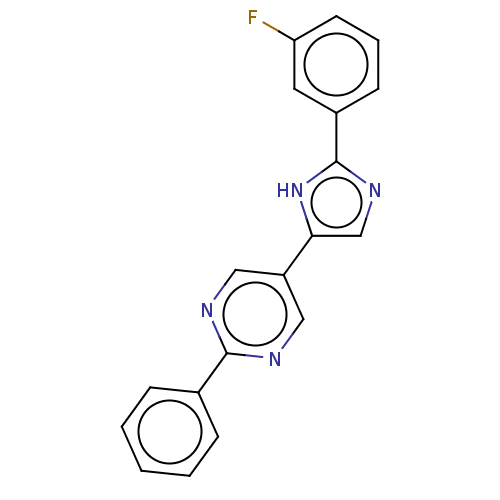
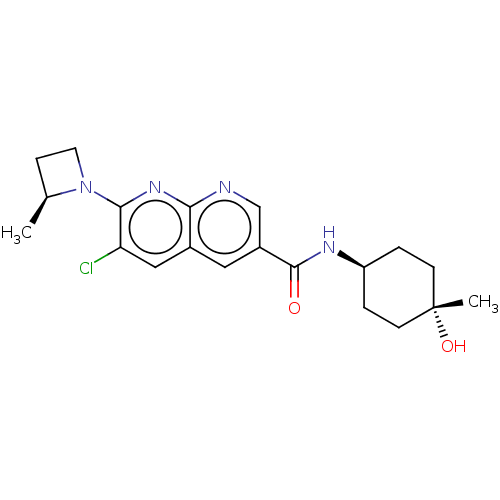
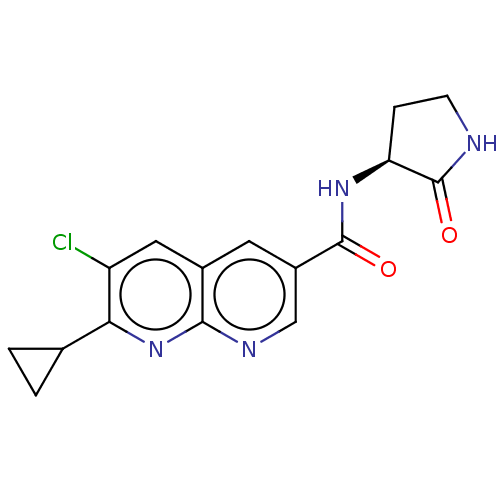
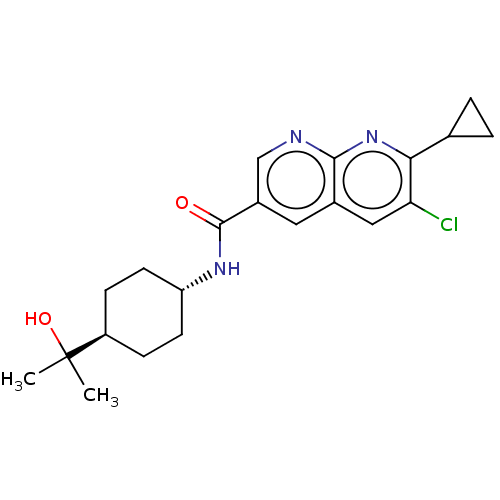
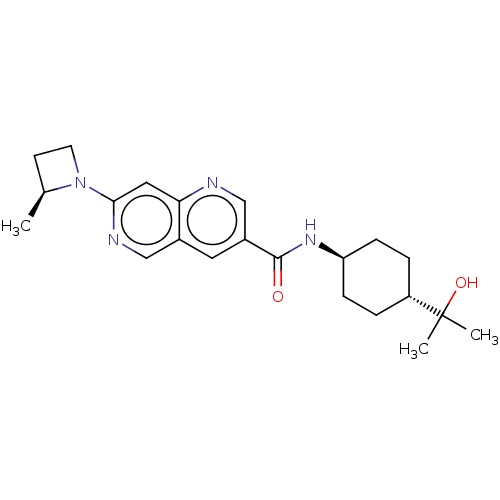
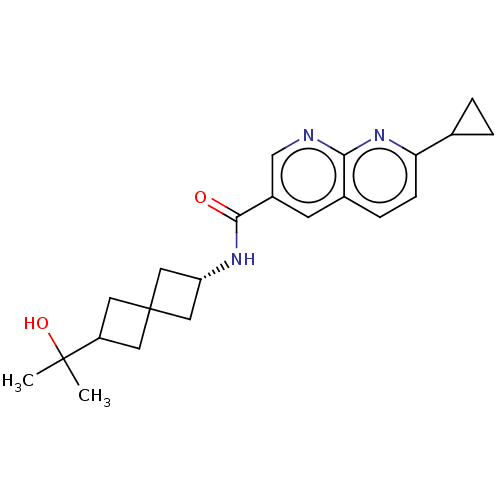
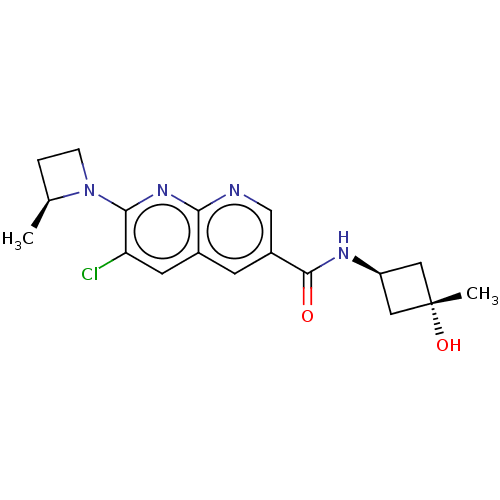
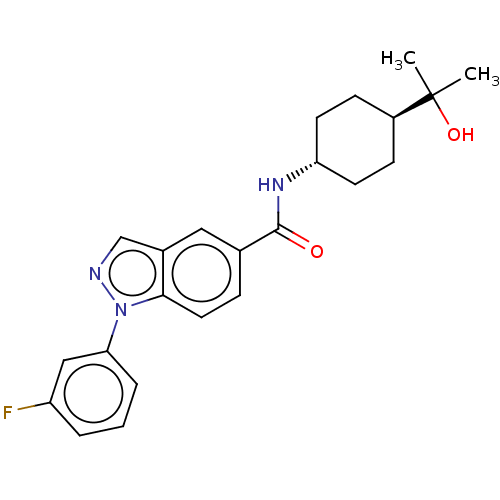
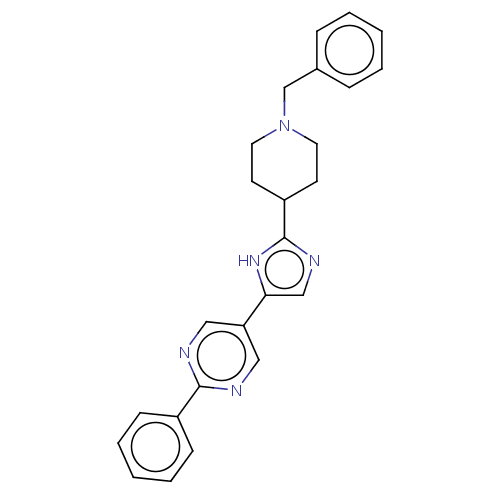
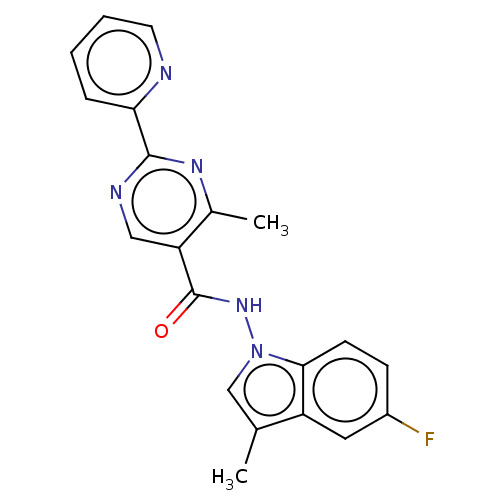
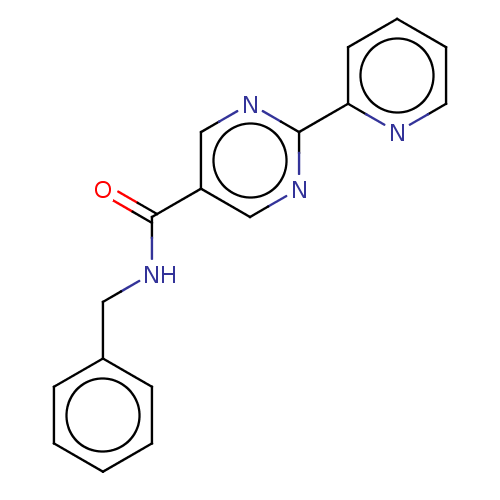
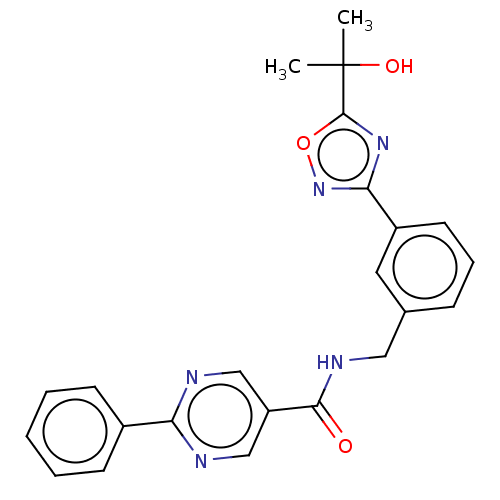
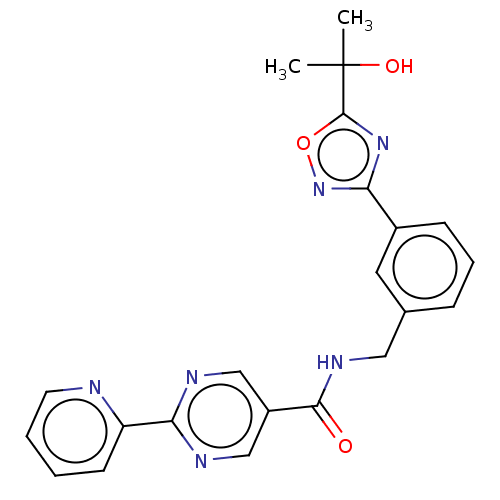
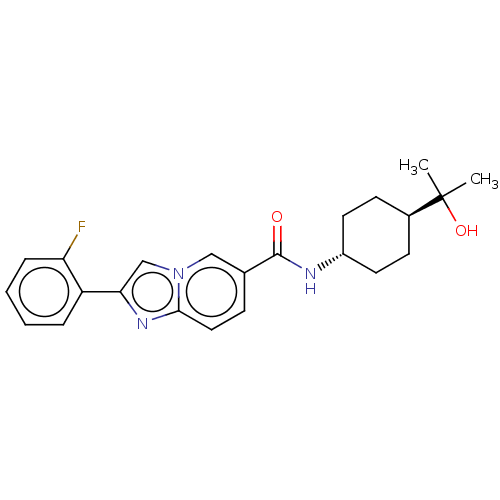
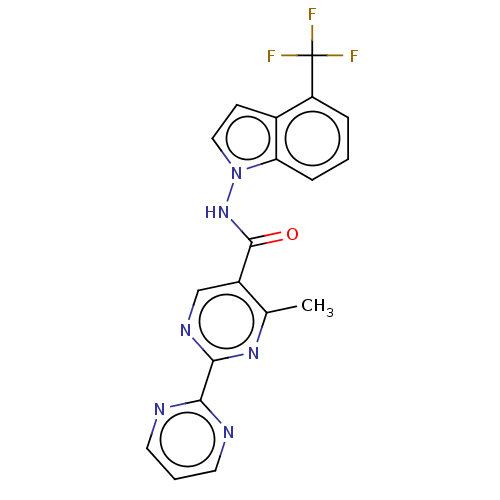
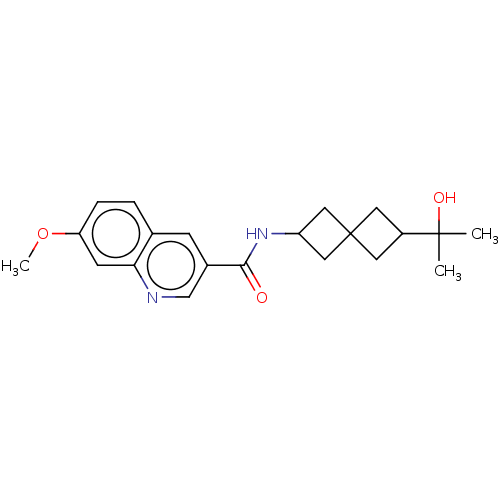
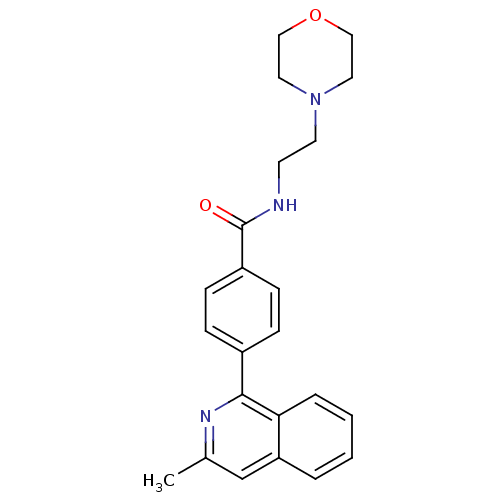
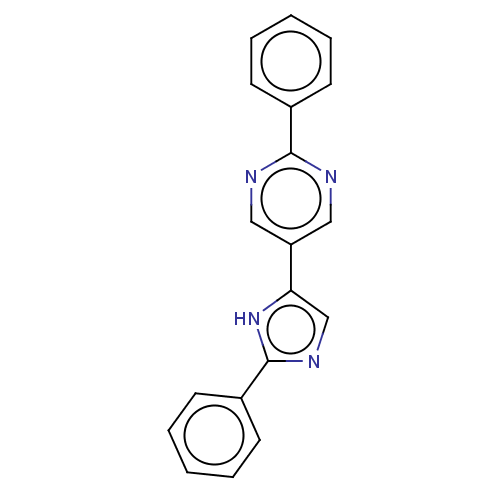
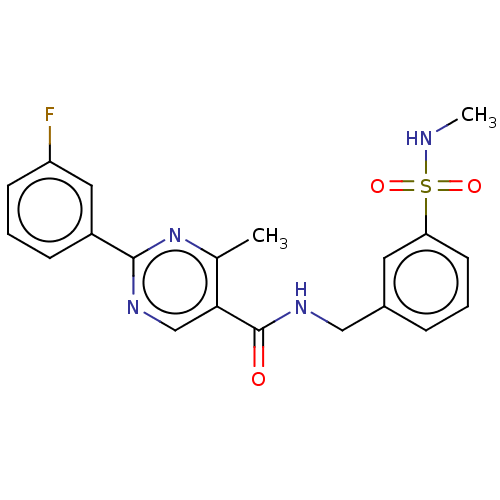
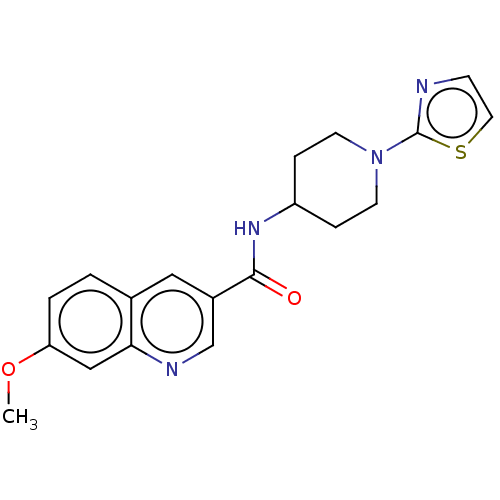
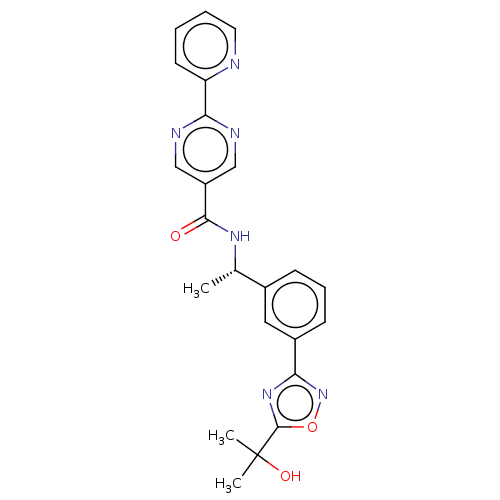
Affinity DataIC50: 0.200nMAssay Description:Inhibition of HPGDSMore data for this Ligand-Target Pair
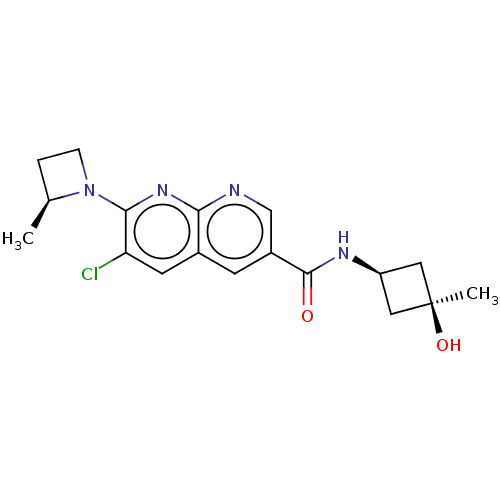
Affinity DataIC50: 2.34nMAssay Description:Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur...More data for this Ligand-Target Pair
Ligand Info
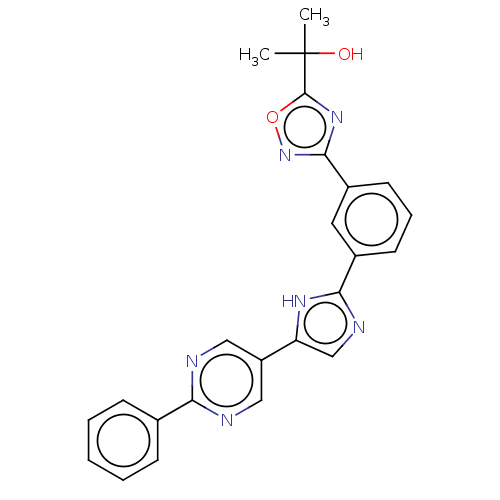
Affinity DataIC50: 3nMAssay Description:Inhibition of HPGDS (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Ligand Info
Ligand Info
Ligand InfoPDB
Ligand Info
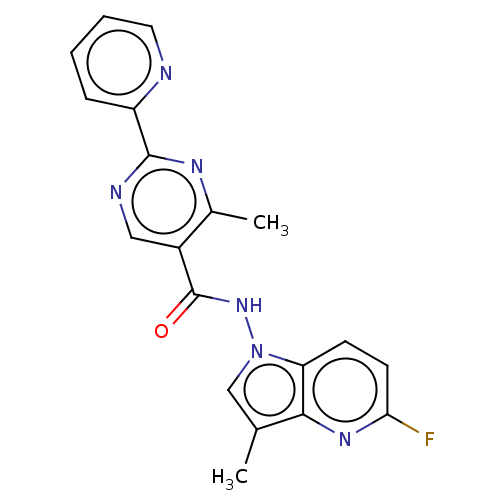
Affinity DataIC50: 4nMpH: 8.0 T: 2°CAssay Description:The assay is carried out by the following steps: 1. Inhibitor screening is performed in 100 mM Tris-HCl, pH 8.0 containing 1 mM GSH, 1 mM MgCl.sub.2,...More data for this Ligand-Target Pair
Ligand Info
Ligand Info
Ligand Info
Ligand Info
Ligand Info
Ligand Info
Ligand Info
Ligand Info
Ligand Info
Ligand Info
Affinity DataIC50: 5.90nMAssay Description:Inhibition of HPGDS (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of HPGDS (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 8.0 T: 2°CAssay Description:The assay is carried out by the following steps: 1. Inhibitor screening is performed in 100 mM Tris-HCl, pH 8.0 containing 1 mM GSH, 1 mM MgCl.sub.2,...More data for this Ligand-Target Pair
Ligand Info
Ligand Info
Ligand Info
Affinity DataIC50: 7nMAssay Description:Inhibition of HPGDS (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 7.10nMAssay Description:Inhibition of HPGDS (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20nMAssay Description:Inhibition of HPGDS (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Inhibition of HPGDS (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of HPGDS (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R...More data for this Ligand-Target Pair
Affinity DataIC50: 8.26nMAssay Description:Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 8.90nMAssay Description:Inhibition of HPGDS (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometryMore data for this Ligand-Target Pair
Ligand InfoPDB
Affinity DataIC50: 9.5nMAssay Description:Inhibition of HPGDS (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 9.70nMAssay Description:Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R...More data for this Ligand-Target Pair
Affinity DataIC50: 9.90nMAssay Description:Inhibition of HPGDS (unknown origin) assessed as reduction in reduction in LPS-induced PGD2 level by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 9.90nMAssay Description:Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of HPGDS (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 11nMpH: 7.4Assay Description:I. Assay Solutions a. Preparation of 0.1M K2HPO4/KH2PO4 buffer (pH 7.4) Prepare 0.1 M KH2PO4 from 1M KH2PO4 (Sigma, Cat# P-8709) ...More data for this Ligand-Target Pair
Ligand Info


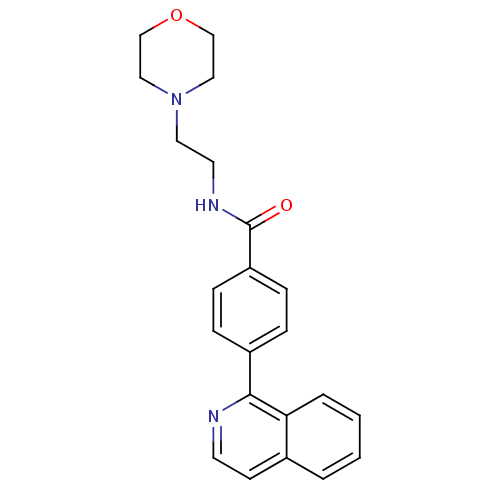
 3D Structure (crystal)
3D Structure (crystal)