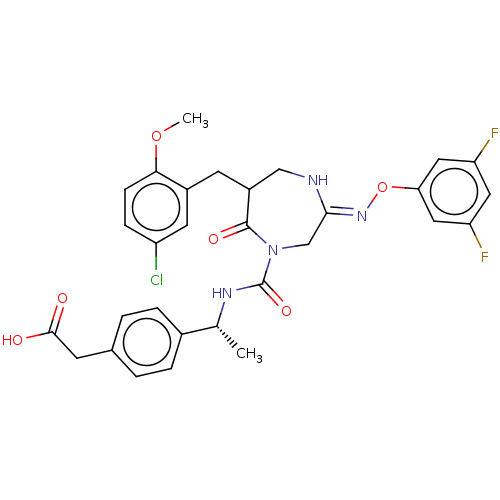
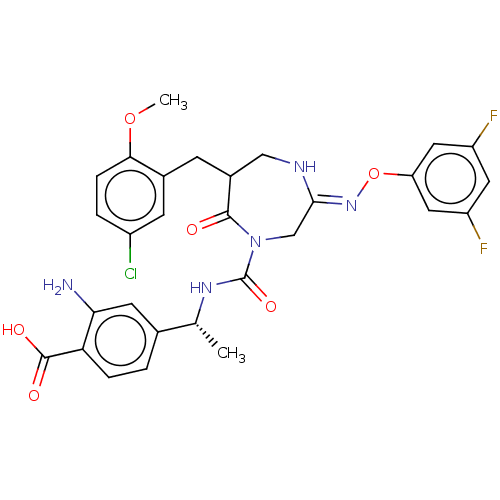
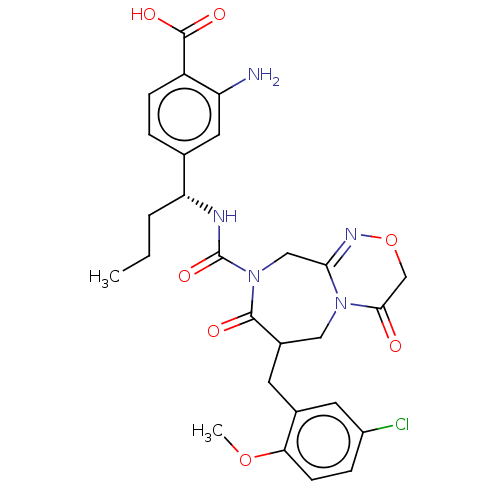
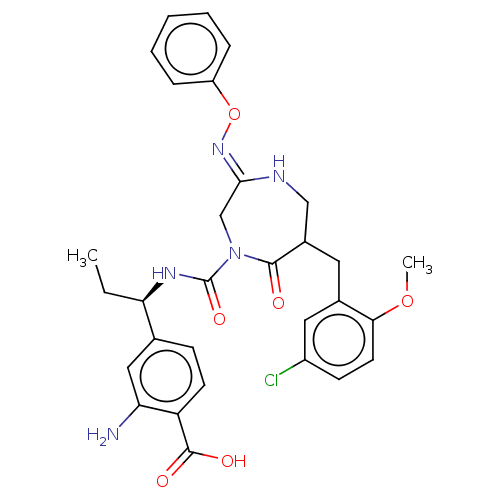
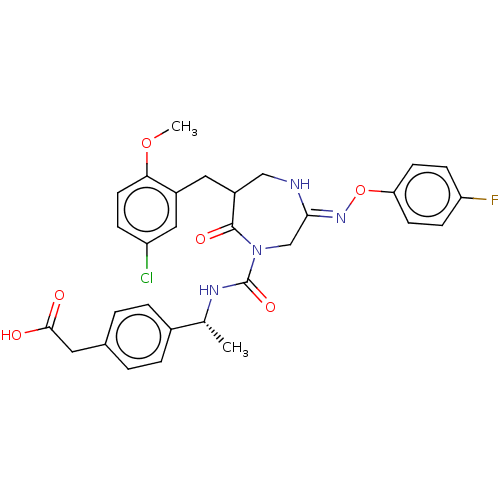
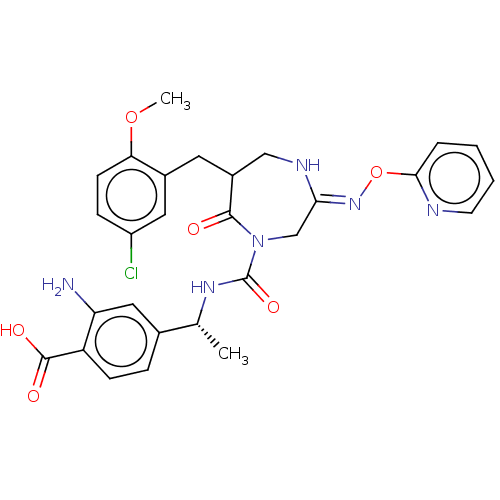
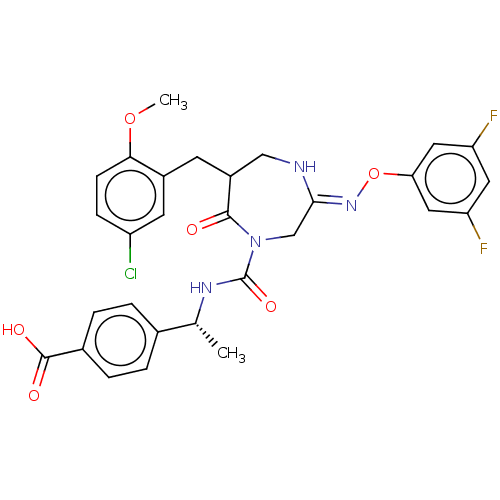
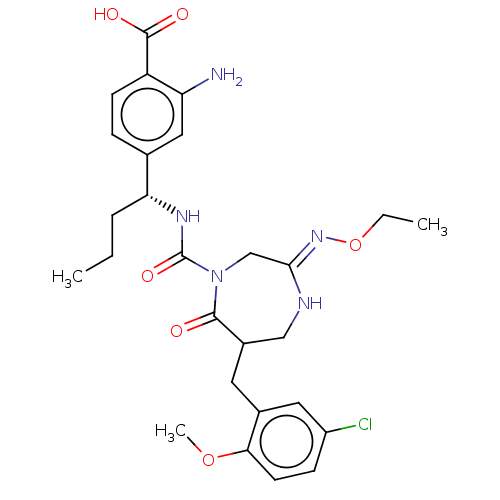
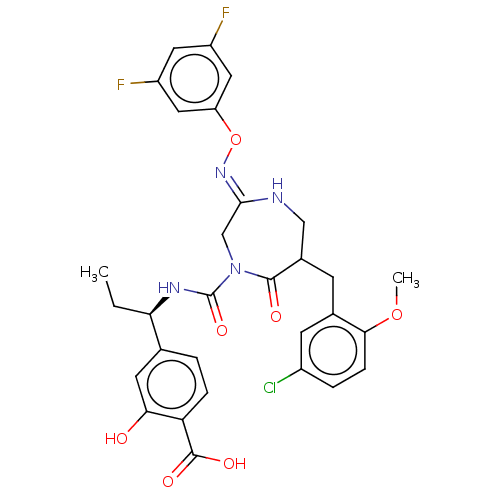
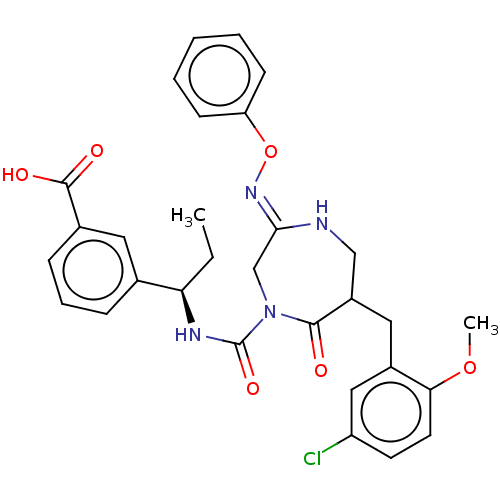
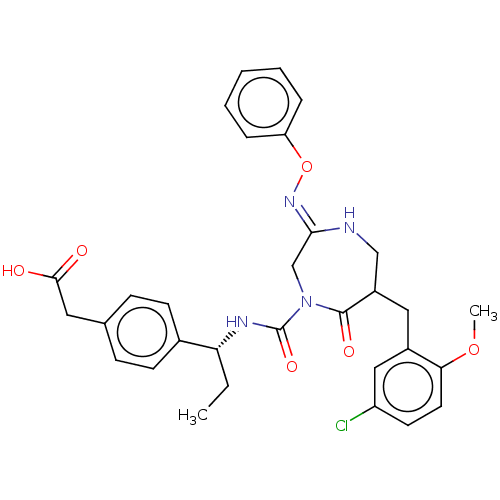
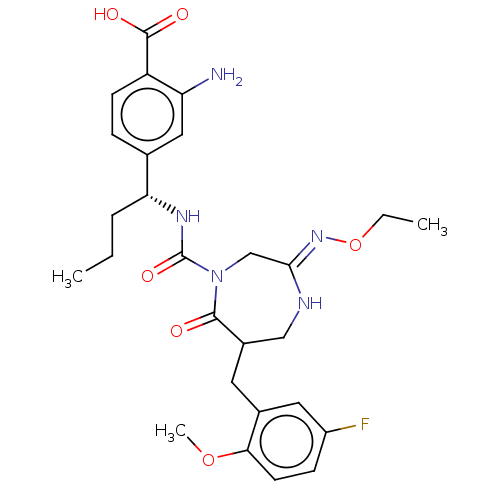
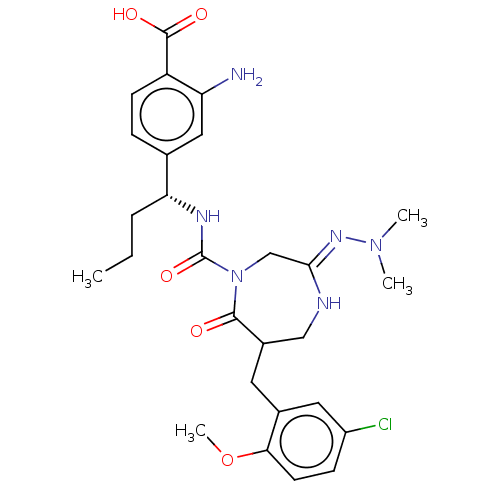
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair