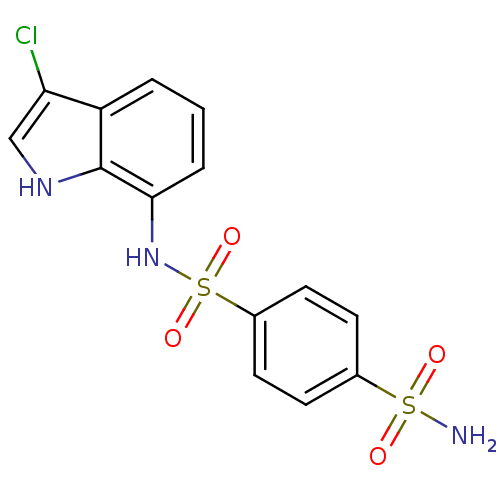
BDBM10890 1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonamide::CHEMBL77517::Compound 6::E7070::Indisulam (IND)::Sulfonamide, 7
SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12
InChI Key InChIKey=SETFNECMODOHTO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 10890
Found 4 hits for monomerid = 10890
Affinity DataKi: 15nMAssay Description:Inhibition of recombinant human carbonic anhydrase-2 by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Inhibition of recombinant human carbonic anhydrase-1 by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 84nMAssay Description:Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair
TargetCarbonic anhydrase, alpha family(Thiomicrospira crunogena (strain XCL-2))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataKi: 273nMAssay Description:Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair