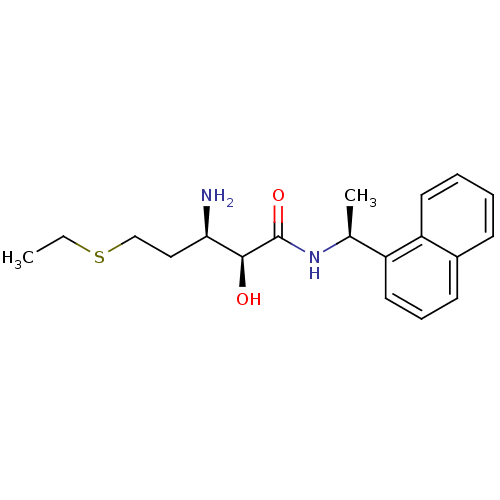
BDBM17589 (2S,3R)-3-amino-5-(ethylsulfanyl)-2-hydroxy-N-[(1S)-1-(naphthalen-1-yl)ethyl]pentanamide::A311263::CHEMBL352943::bestatin-type inhibitor, 4
SMILES CCSCC[C@@H](N)[C@H](O)C(=O)N[C@@H](C)c1cccc2ccccc12
InChI Key InChIKey=AIIOXZPEXXZCML-VHSSKADRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 17589
Found 4 hits for monomerid = 17589
Affinity DataIC50: 22nMpH: 7.4 T: 2°CAssay Description:A coupled enzyme chromogenic assay was developed to measure methionine aminopeptidase activity by monitoring the production of free methionine with L...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibitory activity against human methionine aminopeptidase-2More data for this Ligand-Target Pair
Affinity DataIC50: 370nMAssay Description:Inhibitory activity against human methionine aminopeptidase-1More data for this Ligand-Target Pair
Affinity DataIC50: 370nMpH: 7.4 T: 2°CAssay Description:A coupled enzyme chromogenic assay was developed to measure methionine aminopeptidase activity by monitoring the production of free methionine with L...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)