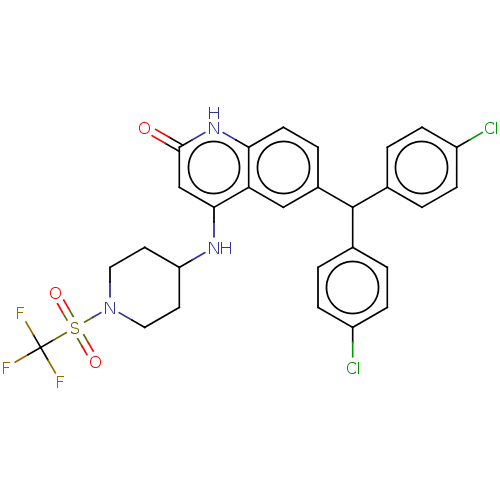
BDBM208271 US9266835, 44
SMILES FC(F)(F)S(=O)(=O)N1CCC(CC1)Nc1cc(=O)[nH]c2ccc(cc12)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1
InChI Key InChIKey=NFALLRQTKNBMAC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 208271
Found 14 hits for monomerid = 208271
Affinity DataEC50: 8nMAssay Description:The following mixtures and buffer solutions were prepared: (a) Buffer 1: HBSS (Mediatech Cat#21-023-CV) with 5 mM HEPES (1 mM stock, Gibco BRL Cat#15...More data for this Ligand-Target Pair
Affinity DataEC50: 5.23E+3nMAssay Description:The following mixtures and buffer solutions were prepared: (a) Buffer 1: HBSS (Mediatech Cat#21-023-CV) with 5 mM HEPES (1 mM stock, Gibco BRL Cat#15...More data for this Ligand-Target Pair
Affinity DataEC50: 1.20nMpH: 7.4 T: 2°CAssay Description:Experimental Procedure CB-1 Membrane Binding: Into Greiner V bottom polypropylene plates, hCB1-CHO-K1 membranes (2 ug/well final concentration) in as...More data for this Ligand-Target Pair
Affinity DataEC50: 53nMpH: 7.4 T: 2°CAssay Description:Experimental Procedure CB-2 Membrane Binding: Into Greiner V bottom polypropylene plates, hCB2-HEK293 membranes (2 ug/well final concentration) in as...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inverse agonist activity at human CB2 receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 10 to 30 mins by LC/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam/testosterone as substrate after 10 to 30 mins by LC/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate after 10 to 30 mins by LC/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Displacement of [3H]-rimonabant from human CB1 receptor expressed in CHO cell membranes after 60 mins by TopCount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO-K1 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 10 to 30 mins by LC/MS analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 8.20nMAssay Description:Inverse agonist activity at human CB1 receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 to 30 mins by LC/MS analysisMore data for this Ligand-Target Pair