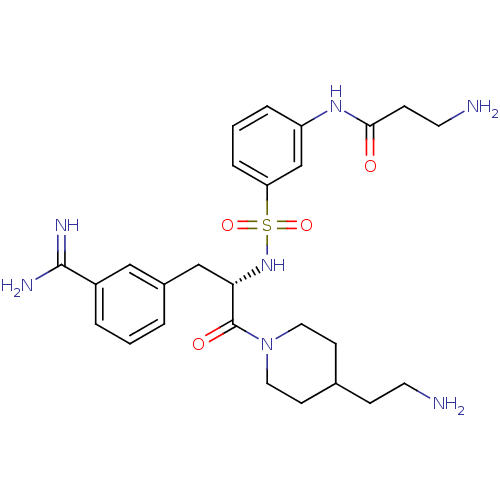
BDBM23917 3-amidinophenylalanine deriv., 59::3-amino-N-(3-{[(2S)-1-[4-(2-aminoethyl)piperidin-1-yl]-3-(3-carbamimidoylphenyl)-1-oxopropan-2-yl]sulfamoyl}phenyl)propanamide::CHEMBL379194
SMILES NCCC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(NC(=O)CCN)c1
InChI Key InChIKey=CEVNTYRWLYLNLF-QHCPKHFHSA-N
Data 10 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 23917
Found 10 hits for monomerid = 23917
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Curacyte Discovery
Curated by ChEMBL
Curacyte Discovery
Curated by ChEMBL
Affinity DataKi: 3.80nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Curacyte Discovery
Curated by ChEMBL
Curacyte Discovery
Curated by ChEMBL
Affinity DataKi: 6.60nMAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nMAssay Description:Inhibition of plasmin (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nMAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Inhibition of uPA (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+4nMAssay Description:Inhibition of factor 10a (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+4nMAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 2.70E+4nMAssay Description:Inhibition of thrombin (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.70E+4nMAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair