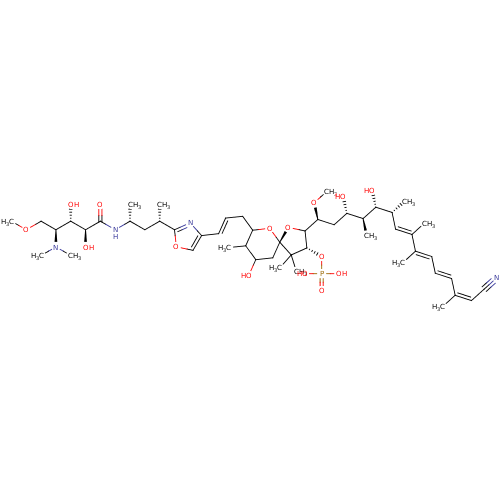
BDBM50061069 CHEMBL384277::Calyculin C
SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)N[C@H](C)C[C@H](C)c1nc(\C=C\CC2O[C@]3(CC(O)C2C)OC([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C
InChI Key InChIKey=MDHVPFKPZGGNLB-CVUGGBTCSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50061069
Found 2 hits for monomerid = 50061069
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Observed inhibition activity of the compounds against protein phosphatases 1 (PP1)More data for this Ligand-Target Pair
TargetSerine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B(Gallus gallus)
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 9.70nMAssay Description:Observed inhibition activity of the compounds against protein phosphatases 2A (PP2A)More data for this Ligand-Target Pair