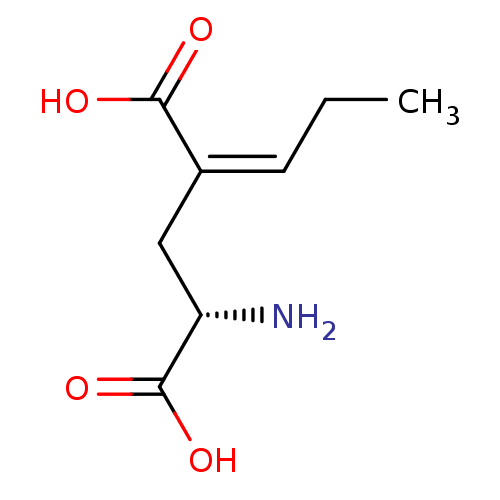
BDBM50091481 (S)-2-Amino-4-propylidene-pentanedioicacid::CHEMBL52295::LY-310683
SMILES CC\C=C(\C[C@H](N)C(O)=O)C(O)=O
InChI Key InChIKey=WKTPEUFZYFCNLY-LMXCLXGDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50091481
Found 4 hits for monomerid = 50091481
Affinity DataKi: 61nMAssay Description:Binding affinity of compound was determined against Ionotropic glutamate receptor ionotropic kainate 1 using cell membranes prepared from HEK293 cell...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+4nMAssay Description:Binding affinity of compound was determined against Ionotropic glutamate receptor ionotropic kainate 1 using cell membranes prepared from HEK293 cell...More data for this Ligand-Target Pair
Affinity DataEC50: 1.42E+4nMAssay Description:Compound was tested for agonistic activity at Glutamate receptor 5 using HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+5nMAssay Description:Compound was tested for agonistic activity at Glutamate receptor 6 using HEK293 cellsMore data for this Ligand-Target Pair