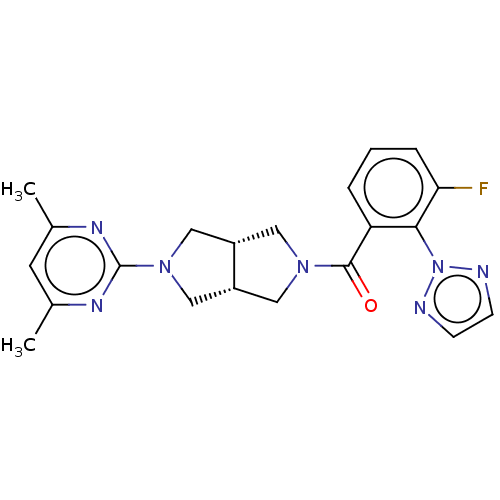
BDBM50092812 CHEMBL3586433
SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cccc(F)c1-n1nccn1
InChI Key InChIKey=RQCIXSIWGGVIAE-IYBDPMFKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50092812
Found 5 hits for monomerid = 50092812
Affinity DataKi: 10nMAssay Description:Binding affinity to rat OX2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding affinity to human OX2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.28E+3nMAssay Description:Binding affinity to human OX1 receptor expressed in CHO cell membranes by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of [3H]-astemizole binding to human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair