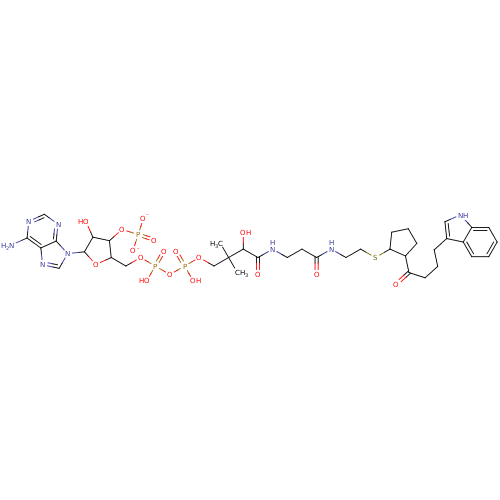
BDBM50101699 Bisubstrate Analogue::[5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy({3-hydroxy-3-[(2-{[2-({2-[4-(1H-indol-3-yl)butanoyl]cyclopentyl}sulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-2,2-dimethylpropoxy})phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl] phosphate
SMILES CC(C)(COP(O)(=O)OP(O)(=O)OCC1OC(C(O)C1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSC1CCCC1C(=O)CCCc1c[nH]c2ccccc12
InChI Key InChIKey=DBHHDSGBFIODKK-UHFFFAOYSA-L
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50101699
Found 2 hits for monomerid = 50101699
TargetSerotonin N-acetyltransferase(Ovis aries)
Johns Hopkins University School Of Medicine
Curated by ChEMBL
Johns Hopkins University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Inhibition of serotonin N-acetyl-transferaseMore data for this Ligand-Target Pair
TargetSerotonin N-acetyltransferase(Ovis aries)
Johns Hopkins University School Of Medicine
Curated by ChEMBL
Johns Hopkins University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:Inhibition of serotonin N-acetyl-transferaseMore data for this Ligand-Target Pair