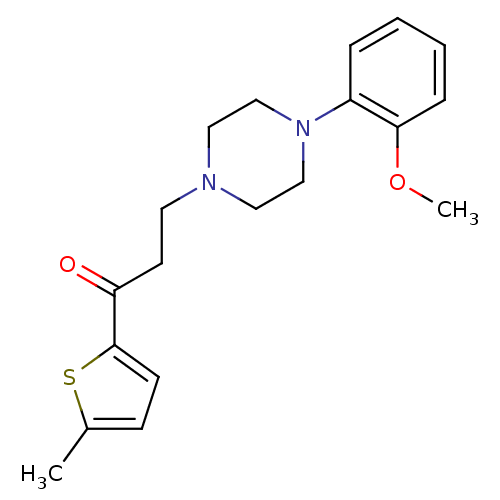
BDBM50102350 3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-(5-methylthiophen-2-yl)propan-1-one::3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-1-(5-methyl-thiophen-2-yl)-propan-1-one::CHEMBL137591
SMILES COc1ccccc1N1CCN(CCC(=O)c2ccc(C)s2)CC1
InChI Key InChIKey=GKFAMQWFMFWCRS-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50102350
Found 4 hits for monomerid = 50102350
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Universidade Federal Da Para£Ba
Curated by ChEMBL
Universidade Federal Da Para£Ba
Curated by ChEMBL
Affinity DataKi: 17.4nMAssay Description:Antagonist activity at 5HT1AMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Universidade Federal Da Para£Ba
Curated by ChEMBL
Universidade Federal Da Para£Ba
Curated by ChEMBL
Affinity DataKi: 17.4nMAssay Description:Binding affinity to 5HT1A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Rattus norvegicus (rat))
Universidad De Navarra
Curated by ChEMBL
Universidad De Navarra
Curated by ChEMBL
Affinity DataKi: 17.5nMAssay Description:Binding affinity to 5-hydroxytryptamine 1A receptor (5-HT 1A receptor, serotonin receptor) from rat cortex using [3H]-8-OH-DPAT as radioligandMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Universidad De Navarra
Curated by ChEMBL
Universidad De Navarra
Curated by ChEMBL
Affinity DataKi: >5.00E+3nMAssay Description:Binding affinity towards Serotonin transporter from rat cortex measured using [3H]-paroxetineMore data for this Ligand-Target Pair