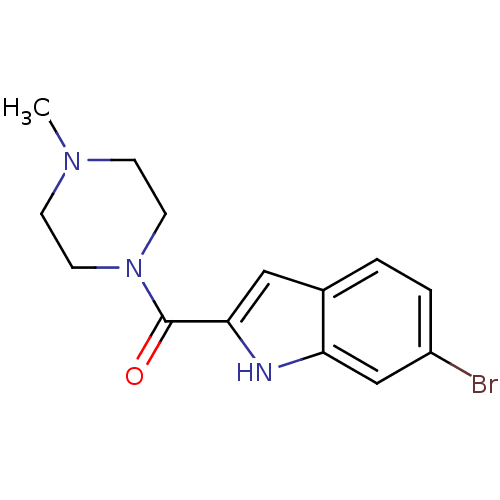
BDBM50133006 (6-Bromo-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)-methanone::(6-bromo-1H-indol-2-yl)(4-methylpiperazin-1-yl)methanone::CHEMBL338001
SMILES CN1CCN(CC1)C(=O)c1cc2ccc(Br)cc2[nH]1
InChI Key InChIKey=YLJOIPRHXDRNJT-UHFFFAOYSA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50133006
Found 2 hits for monomerid = 50133006
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 147nMAssay Description:Displacement of [3H]- histamine from the recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 147nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cellsMore data for this Ligand-Target Pair