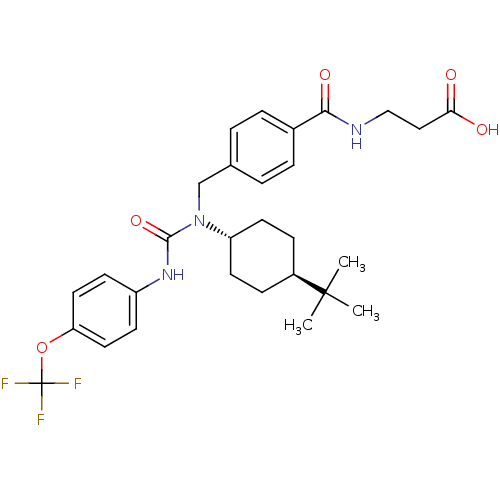
BDBM50144009 3-{4-[1-(4-tert-Butyl-cyclohexyl)-3-(4-trifluoromethoxy-phenyl)-ureidomethyl]-benzoylamino}-propionic acid::CHEMBL62444::trans-3-{4-[1-(4-tert-butylcyclohexyl)-3-(4-trifluoromethoxyphenyl)ureidomethyl]benzoylamino}propionic acid
SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)N(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(OC(F)(F)F)cc1
InChI Key InChIKey=BZXMLCVDKDXRQY-AFARHQOCSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50144009
Found 2 hits for monomerid = 50144009
Affinity DataKi: 63nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 254nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair