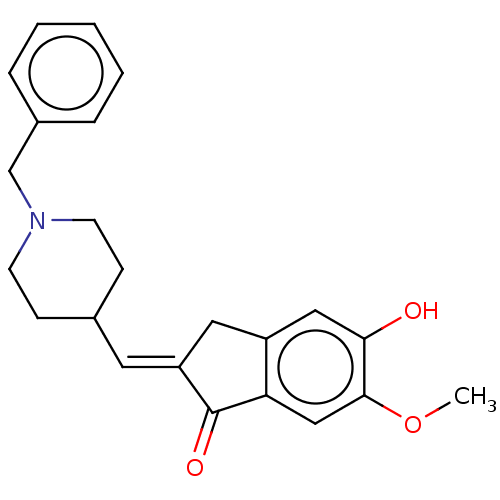
BDBM50182433 CHEMBL3819155
SMILES COc1cc2C(=O)\C(Cc2cc1O)=C\C1CCN(Cc2ccccc2)CC1
InChI Key InChIKey=ZEAXIVSYMLISJH-YBFXNURJSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50182433
Found 4 hits for monomerid = 50182433
Affinity DataKi: 2.82E+3nMAssay Description:Non-competitive inhibition of human erythrocytes AchE using acetylthiocholine iodide as substrate incubated for 20 mins by Lineweaver-Burk plot analy...More data for this Ligand-Target Pair
Affinity DataKi: 1.06E+4nMAssay Description:Non-competitive inhibition of horse serum BuChE using butyrylthiocoline iodide as substrate incubated for 20 mins by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.35E+3nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocoline iodide as substrate incubated for 20 mins by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.54E+3nMAssay Description:Inhibition of human erythrocytes AchE using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman methodMore data for this Ligand-Target Pair