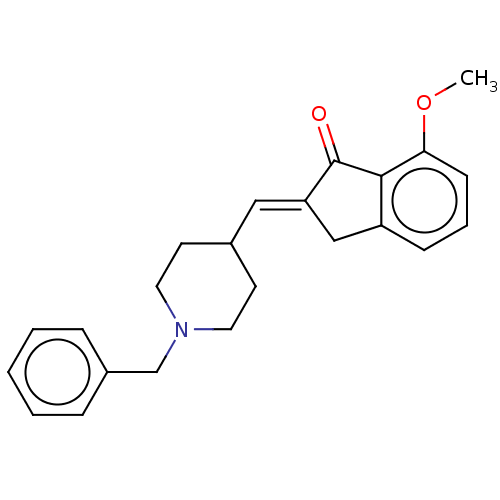
BDBM50182446 CHEMBL3818167
SMILES COc1cccc2C\C(=C/C3CCN(Cc4ccccc4)CC3)C(=O)c12
InChI Key InChIKey=PBMKYXADBLYWBA-XSFVSMFZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50182446
Found 4 hits for monomerid = 50182446
Affinity DataKi: 1.84E+3nMAssay Description:Non-competitive inhibition of human erythrocytes AchE using acetylthiocholine iodide as substrate incubated for 20 mins by Lineweaver-Burk plot analy...More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+3nMAssay Description:Mixed inhibition of horse serum BuChE using butyrylthiocoline iodide as substrate incubated for 20 mins by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+3nMAssay Description:Inhibition of human erythrocytes AchE using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.14E+3nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocoline iodide as substrate incubated for 20 mins by Ellman methodMore data for this Ligand-Target Pair