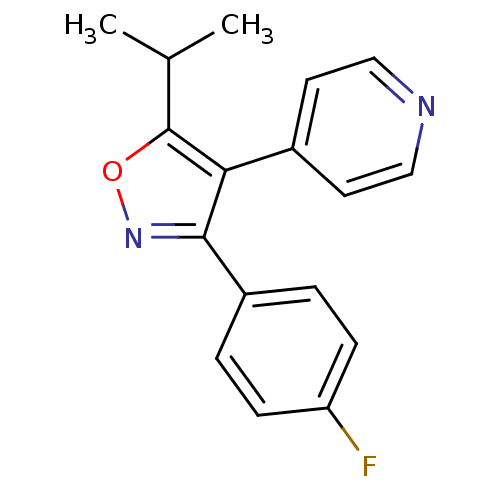
BDBM50206421 3-(4-fluorophenyl)-5-isopropyl-4-(pyridin-4-yl)isoxazole::4-(3-(4-fluorophenyl)-5-isopropylisoxazol-4-yl)pyridine::4-[3-(4-fluorophenyl)-5-isopropylisoxazol-4-yl]pyridine::CHEMBL220227
SMILES CC(C)c1onc(c1-c1ccncc1)-c1ccc(F)cc1
InChI Key InChIKey=BOCQIRYQTWRJMD-UHFFFAOYSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50206421
Found 10 hits for monomerid = 50206421
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Inhibition of JNK3 by non-radioactive immunosorbent assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibition of p38alpha MAPK by non-radioactive immunosorbent assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibition of p38alphaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Eberhard-Karls University
Curated by ChEMBL
Eberhard-Karls University
Curated by ChEMBL
Affinity DataIC50: 1.28E+3nMAssay Description:Inhibition of JNK2More data for this Ligand-Target Pair
TargetCasein kinase I isoform delta(Rattus norvegicus (rat))
Eberhard-Karls University
Curated by ChEMBL
Eberhard-Karls University
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of GST-fused rat CK1delta by Cherenkov counting in presence of 20 uM ATPMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibition of p38alpha by Cherenkov countingMore data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:Inhibition of CK1delta by Cherenkov counting in presence of 20 uM ATPMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Eberhard-Karls University
Curated by ChEMBL
Eberhard-Karls University
Curated by ChEMBL
Affinity DataIC50: 1.28E+3nMAssay Description:Inhibition of JNK2 by Cherenkov counting in presence of 20 uM ATPMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Inhibition of JNK3 by Cherenkov counting in presence of 20 uM ATPMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
Eberhard-Karls-University TüBingen
Curated by ChEMBL
Eberhard-Karls-University TüBingen
Curated by ChEMBL