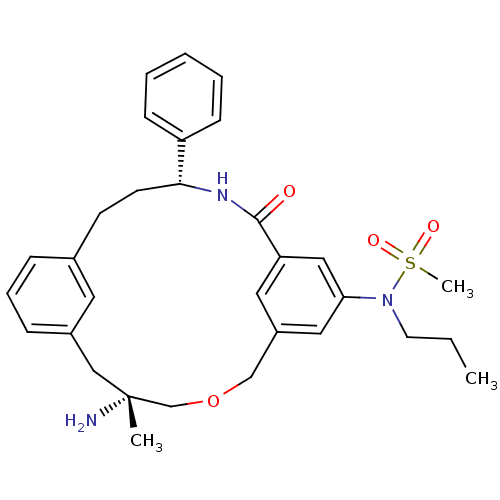
BDBM50212178 CHEMBL248699::N-((5R,14R)-5-amino-5-methyl-16-oxo-14-phenyl-3-oxa-15-aza-tricyclo[15.3.1.1*7,11*]docosa-1(21),7,9,11(22),17,19-hexaen-19-yl)-N-propyl-methanesulfonamide
SMILES CCCN(c1cc2COC[C@](C)(N)Cc3cccc(CC[C@@H](NC(=O)c(c2)c1)c1ccccc1)c3)S(C)(=O)=O
InChI Key InChIKey=PHHMGOKZHVGWDE-BVRKHOPBSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50212178
Found 5 hits for monomerid = 50212178
Affinity DataIC50: 23nMAssay Description:Inhibition of BACE1-mediated sAPP-NF processing in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.44E+5nMAssay Description:Inhibition of reninMore data for this Ligand-Target Pair
Affinity DataIC50: 2.75E+5nMAssay Description:Inhibition of cathepsin DMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of BACE2More data for this Ligand-Target Pair