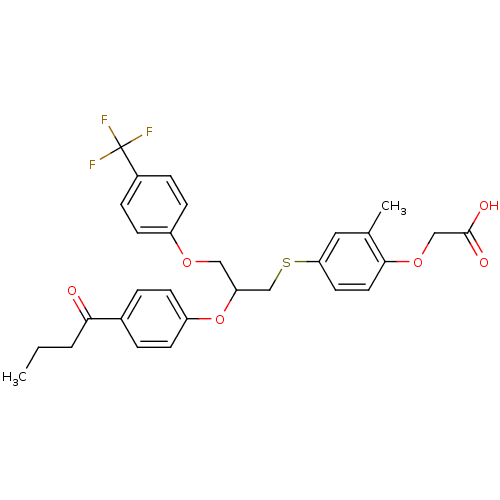
BDBM50213702 2-(4-(2-(4-butyrylphenoxy)-3-(4-(trifluoromethyl)phenoxy)propylthio)-2-methylphenoxy)acetic acid::CHEMBL231527
SMILES CCCC(=O)c1ccc(OC(COc2ccc(cc2)C(F)(F)F)CSc2ccc(OCC(O)=O)c(C)c2)cc1
InChI Key InChIKey=CWQIBOADRQLLDW-UHFFFAOYSA-N
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50213702
Found 3 hits for monomerid = 50213702
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 23.9nMAssay Description:Agonist activity at human PPARdelta receptor by cell based transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Activity at human PPARgamma receptor by cell based transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Activity at human PPARalpha receptor by cell based transactivation assayMore data for this Ligand-Target Pair