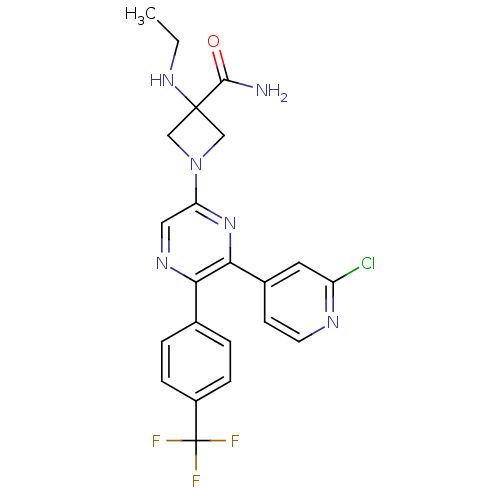
BDBM50260803 1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)phenyl)pyrazin-2-yl)-3-(ethylamino)azetidine-3-carboxamide::CHEMBL498783
SMILES CCNC1(CN(C1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O
InChI Key InChIKey=YSPBHPREQBXDKT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50260803
Found 4 hits for monomerid = 50260803
Affinity DataKi: 1.20nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 2.06nMAssay Description:Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.07nMAssay Description:Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS levelMore data for this Ligand-Target Pair