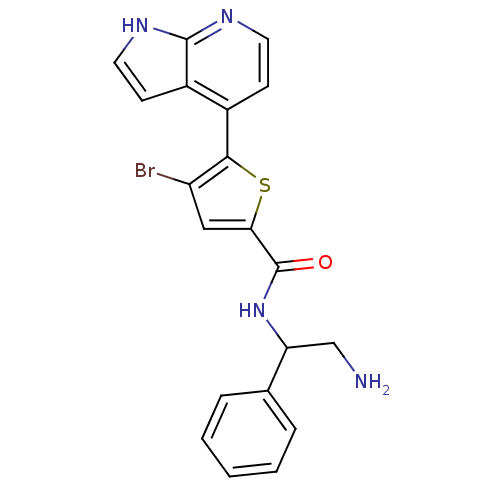
BDBM50278603 (+/-)-N-(2-amino-1-phenylethyl)-4-bromo-5-(1H-pyrrolo[2,3-b]pyridin-4-yl)thiophene-2-carboxamide::CHEMBL512135
SMILES NCC(NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12)c1ccccc1
InChI Key InChIKey=YFDNOSFIXVRVDM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50278603
Found 5 hits for monomerid = 50278603
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
TargetRAC-gamma serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL