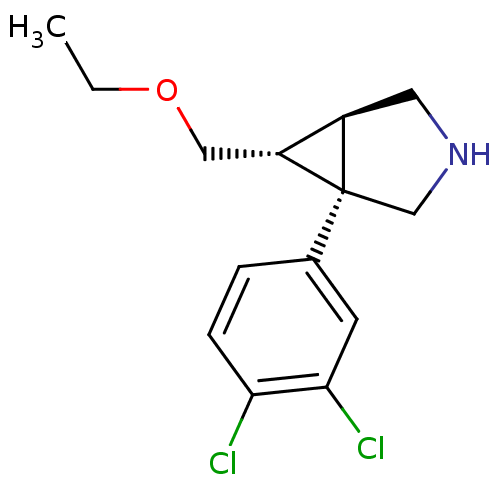
BDBM50308250 (1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)-3-azabicyclo[3.1.0]hexane::CHEMBL598826
SMILES CCOC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1
InChI Key InChIKey=IBZJKEOJOGAMGJ-GYSYKLTISA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50308250
Found 4 hits for monomerid = 50308250
Affinity DataKi: 0.135nMAssay Description:Displacement of [N-methyl-3H]nisoxetine from rat hippocampus NET by filtration binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.851nMAssay Description:Displacement of [3H]citalopram from mouse cortex SERT by filtration binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of histamine H1 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataKi: 1.26E+4nMAssay Description:from Dfrom ifrom sfrom pfrom lfrom afrom cfrom efrom mfrom efrom nfrom tfrom from ofrom ffrom from [from 3from Hfrom ]from dfrom ofrom ffrom efrom tf...More data for this Ligand-Target Pair