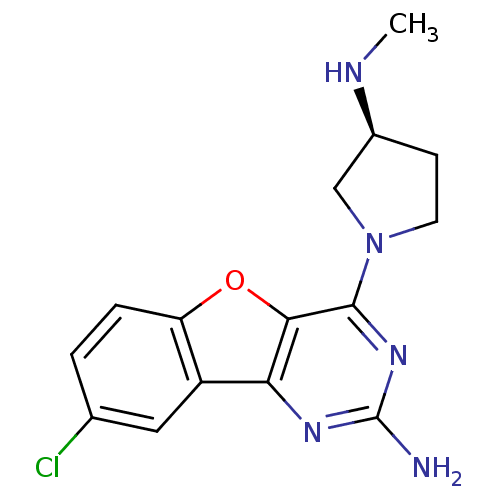
BDBM50315349 (S)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)benzofuro[3,2-d]pyrimidin-2-amine::CHEMBL1091875
SMILES CN[C@H]1CCN(C1)c1nc(N)nc2c3cc(Cl)ccc3oc12
InChI Key InChIKey=POXFWGOYGQTRMD-VIFPVBQESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50315349
Found 3 hits for monomerid = 50315349
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inverse agonist activity at human histamine H4 receptor assessed as inhibition of [35S]GTPgammaS binding after 15 mins by scintillation proximity ass...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assayMore data for this Ligand-Target Pair